Sexually Transmitted Infections (STIs) and Diseases (STDs): A Growing Epidemic
Online Continuing Education Course

Course Description
Nursing CEU course to care for patients at risk or with sexually transmitted infections. Recognize signs and symptoms for STIs; understand prevention based on the National Strategic Plan; and review treatment for chlamydia, gonorrhea, HPV, and syphilis. Covers challenges in controlling the spread of STIs and the impact of COVID-19.
Course Price: $12.00
Original Price: $24.00
Exclusive 50% Discount
Contact Hours: 3
Pharmacotherapeutic Hours: 0.5
Course updated on
July 6, 2022
"I learned many new things. It also changed my thinking on information I previously thought was correct." - Douglas, RN in Hawaii
"The course provided updates regarding STD evaluation and treatment. I also learned that laws for treatment with EPT are different and not allowed in some states. The course was excellent." - Shirly, NP in California
"Thanks for a very interesting class! A bit of an eye opener for sure. We are seeing this more and more in the field!" - Gary, RN in California
"This course was better than I expected and flowed well through the information." - Patricia, RN in Georgia
Sexually Transmitted Infections (STIs) and Diseases (STDs): A Growing Epidemic
Copyright © 2022 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will be prepared to provide evidence-based care for patients with those sexually transmitted infections (STIs) identified as reaching epidemic levels in the United States. Specific learning objectives to address potential knowledge gaps include:
- Summarize the status of STIs in the United States.
- Discuss the STI National Strategic Plan.
- Analyze the screening, diagnosis, and care of the patient with a major STI identified as affecting population health.
- Identify the challenges in controlling the spread of STIs
- Describe the impact of COVID-19 on the development, identification, and treatment of STIs.
TABLE OF CONTENTS
SCOPE OF THE STI PROBLEM
The United States is currently facing a public health crisis due to rising rates of sexually transmitted infections (STIs). During the time period from 2014 to 2018, the rates of these STIs rose dramatically. Primary and secondary syphilis rose 71%, while the rate of increase for congenital syphilis was 185%, gonorrhea 63%, and chlamydia 19% (U.S. DHHS, 2019). Despite a vaccine that targets the strains of human papilloma virus (HPV) that can cause cancer, there are also approximately 35,000 new cases of cervical cancer diagnosed each year (U.S. DHHS, 2020).
According to the Centers for Disease Control and Prevention (CDC), two decades ago the rates of gonorrhea and syphilis had dropped significantly, and new diagnostic techniques made diagnosis of chlamydia easier. In recent years, however, there has been an increase in the rate of STIs that has reached the level of a public health crisis (U.S. DHHS, 2020):
- Chlamydia was the most commonly reported STD/STI in the United States in 2018, with nearly 1.8 million reported cases.
- In the 5-year period from 2014–2018, gonorrhea showed a 63% increase, with 583,405 new cases reported in 2018.
- Syphilis showed a 71% increase from 2014–2018, with 30,644 newly diagnosed cases reported in 2017, up from 17,375 in 2013.
- HPV, while not a reportable disease, infects approximately 13 million adolescents and adults annually.
(Workowski et al., 2021; CDC, 2021a, 2021d)
NATIONALLY REPORTABLE STDs
Healthcare providers are required to report certain diseases to their local health department. The local health department then notifies the state health department, which in turn notifies the CDC. Nationally, reportable STDs are chlamydia, chancroid, gonorrhea, hepatitis B, HIV, and syphilis (Boskey, 2021).
According to the CDC, 20% of the U. S. population had an STI (about 68 million infections) in 2018, with nearly half occurring in adolescents and young adults ages 15–24 years. Among those STDs/STIs whose rates are increasing, CDC statistics show a higher incidence of gonorrhea and syphilis in men (especially men who have sex with men [MSM]) than women and a higher incidence of chlamydia in women than men in newly reported cases (CDC, 2021l, 2021d).
TERMINOLOGY
The terms STDs and STIs are often used interchangeably, although they have different meanings. STI refers to infection with a pathogen that is transmitted through sexual contact, while STD refers to the recognizable disease that develops as a result of the STI. The STI Nation Strategic Plan prefers the term STI and emphasizes the aim of preventing or treating infections before they become STDs. Additionally, individuals may carry an infection but not have a disease (HHS, 2020).
The term venereal disease (VD) has been replaced by the term STD.
Health Consequences of STIs
Because many STIs do not produce symptoms, they can go unnoticed and unknowingly spread to others. A diagnosis of an STI may not be made until serious health consequences have occurred. Social stigma may also keep individuals from seeking care for STIs. Other factors that contribute to the current STI/STD epidemic include poverty, lack of education, and inequalities in access to healthcare (U.S. DHHS, 2020).
Without proper medical care, STIs can result in negative health consequences, such as pelvic inflammatory disease (PID), pelvic pain, infertility, ectopic pregnancy, miscarriage, poor neonatal consequences, and, in the case of HPV, cancer (U.S. DHHS, 2020). Moreover, individuals with chlamydia, gonorrhea, and syphilis are more likely to contract human immunodeficiency virus (HIV) and to transmit the virus to their sex partners.
Relevance of STIs to All Clinical Specialties
Clinicians who work in a variety of healthcare setting may encounter patients with an STI as a presenting symptom or while caring for patients’ other health problems. Therefore, all clinicians should be able to recognize high-risk behaviors and signs and symptoms of STIs, provide basic STI education, deliver STI care following standard recommendations, and know when to refer patients for specialized care.
The following are examples of non-STD clinical settings in which clinicians may encounter patients with STIs/STDs:
- A history and physical examination of a female patient in a fertility clinic may reveal that fertility issues are related to untreated gonorrhea.
- A patient admitted to a medical unit for persistent abdominal pain and urinary tract infections may be diagnosed with chlamydia.
- A preadmission history and physical examination for a patient scheduled for a total knee arthroplasty may reveal that the patient has genital lesions that require treatment prior to surgery.
- A history on an adult during a routine annual examination may reveal multiple sex partners and inconsistent use of condoms, requiring STI testing and education about safe sex practices.
- An adolescent in high school may share symptoms of an STI with the school nurse.
- A school-aged child in a pediatric clinic may have symptoms suggesting an STI and sexual abuse.
NATIONAL STRATEGIC PLAN TO ADDRESS STIs
To combat the considerable rise in STIs, the U.S. Department of Health and Human Services (HHS) developed a 5-year plan aimed at preventing and treating STIs in America. The purpose of the plan is to provide guidance at the national level to halt the increase in STIs. The plan also recognizes that certain demographics are overly affected by the upward trend in STIs. Although this is a national plan, it emphasizes the importance of individualizing the plan to stakeholder populations and resources (U.S. DHHS, 2019).
Part of the problem noted in several national reports is the lack of a coordinated approach in addressing the prevention and treatment of STIs. Many public health agencies at the local and state levels do not have sufficient resources to address the STI epidemic. The STI National Strategic Plan provides a coordinated framework to address the STI epidemic for government and nongovernment parties at national, state, and local levels (U.S. DHHS, 2020).
Strategic Plan Goals
The STI National Strategic Plan focuses on chlamydia, gonorrhea, syphilis, and HPV. Of the 30 or more STIs, these four carry the greatest rates of morbidity, have the most significant STI burden, and create the highest national health impact. The focus on HPV, gonorrhea, and syphilis also lines up with the World Health Organization’s focus in their 2016–2021 global strategy.
The plan has five main goals to provide all individuals with high-quality STI prevention, screening, and treatment free from stigma and discrimination:
- Prevent new STIs
- Improve the health of people by reducing adverse outcomes of STIs
- Accelerate progress in STI research, technology, and innovation
- Reduce STI-related health disparities and health inequities
- Achieve integrated, coordinated efforts that address the STI epidemic
(U.S. DHHS, 2019)
The STI National Strategic Plan emphasizes that no one goal is more important or carries a higher priority than the others.
PREVENTING NEW STIs
One goal of the STI National Strategic Plan is to prevent the spread of STIs through primary prevention—that is, prevention of STIs from occurring in susceptible populations. To be effective in high-risk populations, prevention education programs should demonstrate cultural, linguistic, and age sensitivity. Moreover, education should be presented in a manner that reduces stigma associated with STIs and include information on the importance of STI testing in high-risk populations.
REDUCING ADVERSE OUTCOMES OF STIs
Another goal of the STI National Strategic Plan uses secondary and tertiary prevention to stop STIs from developing into STDs and to provide treatment when an STD develops. This goal involves better, earlier, and more widespread screening to identify and treat those with STIs to prevent progression to an STD and halt the potential spread to others. Moreover, having an STI places an individual at risk for HIV.
When individuals present for treatment of an STI, pregnancy testing, or birth control, a discussion of testing for other STIs should take place. Electronic health records with clinical decision support systems can help providers identify at-risk individuals who require further testing or care (U.S. DHHS, 2019).
This goal also calls for education of healthcare providers and the workforce in various settings about STI prevention, screening, diagnosis, and treatment.
ACCELERATING RESEARCH, TECHNOLOGY, AND INNOVATION
A third goal of the STI National Strategic Plan addresses the use of research, technology, and innovation to improve and develop rapid point-of-care diagnostic testing, self-specimen collection, vaccinations, and treatment of STIs as well as fighting antimicrobial resistance and promoting antimicrobial stewardship (HHS, 2019).
THE ADVANTAGE OF RAPID TESTS
To prevent the spread of STIs, it is ideal to diagnose and treat diseases during the patient’s same visit. This immediacy safeguards against patients not returning for a second visit and reduces the time during which patients are infectious, thus decreasing the spread of the infection.
Rapid tests that can be performed in less than 30 minutes are available for syphilis, gonorrhea, chlamydia, HIV, and genital herpes. There are also several syphilis rapid tests that can return results within 15 minutes and can be performed and interpreted by nonlaboratory personnel. One test involves obtaining whole blood via fingerstick and then applying it to the testing device (Fakile et al., 2019).
REDUCING HEALTH DISPARITIES AND INEQUITIES
A fourth goal addresses health disparities and inequities. Some populations do not have access to STI prevention resources, while others face discrimination or social stigmas related to STIs or are more prone to high-risk behaviors. Still other groups may have a mistrust of the medical system. Certain racial, ethnic, sexual, and gender populations also face higher rates of STIs. Other factors that are related to a higher rate of STIs include poverty, a lack of health insurance, and employment issues. Healthcare providers must work to ensure universal access to care in a welcoming, culturally sensitive, and trauma-informed manner.
The STI National Strategic Plan addresses the disproportionate increase in STIs that has affected certain “priority” populations, including adolescents and young adults, men having sex with men, pregnant women, and certain racial and ethnic minorities (U.S. DHHS, 2019).
Adolescents and Young Adults
Adolescents and young adults acquire STIs at disproportionately high rates. While they represent just over 25% of the sexually active, those ages 15–24 years account for half of the 26 million new STIs that occur in the United States each year. Approximately 25% of sexually active adolescent females has an STD (CDC, 2021b, 2020a).
Certain activities prevalent in the adolescent population put them at higher risk for exposure to STIs: new or multiple or anonymous sexual partners, concurrent illicit drug use (especially methamphetamines), MSM, and HIV-positive status (CDC, 2019). Rates of oral and anal sex are also rising among adolescents wishing to avoid pregnancy and the loss of virginity, and these practices may lead to oral, throat, and anorectal STIs/STDs.
Men Who Have Sex with Men
Men who have sex with men accounted for more than half the cases of primary and secondary syphilis in 2018. An elevated STI burden is of concern because it may indicate high risk for HIV infection. The high incidence of infection among MSM may be related to factors such as the number of sex partners, rate of partner exchange, and frequency of condomless sex. Furthermore, experiences of stigma are associated with increased sexual risk behavior among MSM.
PATIENTS WITH NONBINARY GENDER IDENTITIES
Transgender is an umbrella term for persons whose gender identity or expression (masculine, feminine, other) is different from their sex (male, female) at birth. Gender identity refers to one’s internal understanding of one’s own gender or the gender with which a person identifies. Gender expression is a term used to describe people’s outward presentation of their gender.
Gender identity and sexual orientation are different facets of identity. Everyone has a gender identity and a sexual orientation, but a person’s gender does not determine a person’s sexual orientation. Transgender people may identify as heterosexual, homosexual, bisexual, or none of the above.
With transgender patients, it is important to ask questions necessary to assess the pertinent issue but to avoid unrelated probing. For example: “To help assess your health risks, can you tell me about any history you have had with hormone use?”
When providing care to transgender patients, healthcare providers should communicate in a way that promotes respect and makes transgender individuals feel comfortable and welcome:
- Avoid the use of gendered titles such as “Sir” or “Ma’am.” Instead of Mr. or Ms., patients may wish to be addressed as Mx. (pronounced with a “ks” or “x” sound at the end).
- Ask patients for information such as pronouns, preferred name, and gender identity. Pronouns may include he/his/him, she/hers/her, or a range of options for nonbinary transgender patients, such as they/their/them, ze, sie, hir, co, and ey. Always respect the patient’s pronouns and apologize if the wrong pronouns are used by mistake.
- Always ask for clarification about what a patient would like to be called or how the patient would like to be addressed. Apologize if referring to a patient in a way that inadvertently seemed offensive.
- Ask patients what terms they use to refer to their anatomy and mirror those terms during the patient interaction. Transgender patients may experience gender dysphoria and may not be comfortable with traditional terms for body parts.
- Do not make assumptions about patients’ sexual orientations, gender identities, beliefs, or concerns based on physical characteristics such as clothing, tone of voice, or perceived femininity/masculinity.
- Do not ask patients questions about sexual orientation or gender identity that are not material to their care or treatment.
(CDC, 2021r; Ness, 2020)
Pregnant Women
From 2014 to 2108, there was a 185% rate increase in congenital syphilis in pregnant women, leading to stillbirths and infant deaths. When treating a woman for an STD/STI, it is important to know if she could be pregnant. Therefore, a pregnancy test is commonly added to other STD/STI tests for women in their reproductive years.
Racial and Ethnic Minorities
Racial and ethnic minorities may face barriers in accessing healthcare or be uninsured. STIs have a greater impact on certain subgroups of these priority populations, including Black, American Indian/Alaska Native, and Hispanic populations.
Moreover, higher rates of STIs can be seen in the southern and western parts of the United States. The rates of primary, secondary, and congenital syphilis are greatest in the western regions, while chlamydia is highest in the southern region. The rates of gonorrhea are highest in the southern and western regions, while HPV vaccination rates are lowest in the south (U.S. DHHS, 2020).
ACHIEVING COORDINATION OF CARE
A fifth goal of the STI National Strategic Plan relates to providing coordination among various levels of government and across programs to address the STI epidemic to improve quality of care and preventative services. This coordinated approach encourages a sharing of ideas and strategies and promotes collaboration to improve health outcomes. Coordinated care also involves the collection and use of data to monitor and evaluate progress (U.S. DHHS, 2019).
DISEASE-SPECIFIC DIAGNOSIS, TREATMENT, AND PREVENTION
Most STIs are a threat to both the health of the patient and the health of the community, and treatment is always recommended. STIs present with a variety of syndromes, but their treatment depends on the specific agent causing the problems.
The following are the current recommended treatments for chlamydia, gonorrhea, syphilis, and HPV, the STIs recognized by the National Strategic Plan as reaching epidemic proportions. The drugs and administration regimens offered here are examples of current recommendations based on CDC guidelines, but actual treatments must be tailored to the specific patient.
(For a link to the most current CDC treatment recommendations, see “Resources” at the end of this course.)
TWO TREATMENT PRINCIPLES
1. Treat As Soon As Possible
Early care for STIs can shorten the time for screening, diagnosing, treating, and educating. Early treatment is based on the patient’s symptoms, the STI risk assessment from the medical history, and the signs observed during the physical examination. Rapid laboratory test results may be available for use in diagnosis, but when tests take a day or more to produce results, presumptive treatment is often started before a diagnosis has been verified. When available, medications are given in single-dose regimens that are dispensed during the initial visit, and the patient is asked to take the medicine immediately.
The aggressive treatment of STIs may seem at odds with slowing the development of antibiotic-resistant microbes. The quick treatment of STIs, however, is driven by public health concerns. When patients seek care, a follow-up visit for treatment cannot be guaranteed. Sometimes, the best assurance that diseases will not be spread further is treatment at the first visit.
2. Extend Treatments Beyond the Individual Patient
The patient is instructed to avoid sexual contact for the appropriate period of time depending on the infection and the treatment regimen. The patient is also advised to avoid sexual contact with partners until the partners have also been treated. The patient is instructed to notify recent sexual partners about the patient’s STI, and the partners are encouraged to have STI testing. The patient is assured that the confidentiality of all parties’ medical records will be strictly preserved.
(Mayo Clinic, 2021a)
Chlamydial Infections
Of the reportable diseases, chlamydial infections were the most common STDs/STIs in the United States in 2019. Chlamydial infections were more prevalent among young women, with 3,728.1 cases per 100,000, an increase of 10% from 2015. The rates among males increased 32.1% from 2015 to 2019, possibly a result of increased availability of urine testing and extragenital screening and increased transmission in males. Both genders show a decrease in the occurrence of chlamydia as age increases. However, many cases go undiagnosed because most people with chlamydia are asymptomatic and do not seek testing. Chlamydia can be prevented by using male latex condoms, abstinence, and monogamy (Workowski et al., 2021; CDC, 2021e).
CLINICAL MANIFESTATIONS
Chlamydial infections produce genital or lower urinary tract infections. After a person acquires a chlamydial infection, there is an incubation period of several weeks before the organism produces symptoms. But chlamydial infections are frequently asymptomatic; only 10% of men and 5%–30% of women with laboratory-confirmed chlamydial infection develop symptoms, potentially causing reinfection of sexual partners (CDC, 2021e).
In men, symptomatic chlamydial infections show up as urethritis or epididymitis, with pain and swelling around a testicle. Chlamydial urethritis produces a mucoid, mucopurulent, or purulent discharge; inflammation; pain on urination; and urethral itching.
In women, symptomatic chlamydial infections show up most often as cervicitis. Chlamydial cervicitis produces a mucopurulent or purulent discharge in the endocervical canal; pain on urination or during intercourse; and vaginal bleeding after intercourse or between periods. Alternately, chlamydial cervicitis sometimes gives no other findings than a friable cervix on speculum examination.
Untreated, chlamydial cervicitis can spread and cause PID, which can then lead to infertility or ectopic pregnancies; and women may eventually develop lower abdominal pain, lower back pain, nausea and vomiting, fever, painful sex, and breakthrough bleeding during the menstrual cycle. During vaginal births, pregnant women can pass chlamydial infections to their newborns. The resulting infection can cause pneumonia and conjunctivitis in the infant (CDC, 2021e).
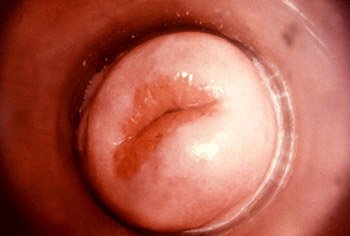
Colposcopic view of a female patient’s cervix with signs of erosion and erythema due to a Chlamydia trachomatis infection. (Source: CDC/Dr. Lourdes Fraw, Jim Pledger, Public Health Image Library.)
SCREENING AND DIAGNOSIS OF CHLAMYDIA
The CDC recommends annual chlamydia screening for all sexually active females younger than 25 and for women 25 years and older with risk factors such as a new sex partner or multiple partners. Pregnant women should be screened during their first prenatal care visit (CDC, 2021f). For women, screening for asymptomatic cases is important because routine screening has been shown to reduce the number of cases of PID.
To make widespread screening practical, urine specimens and self-obtained vaginal swabs can be used because they provide effective samples for chlamydia screening tests, although examinations by a licensed provider are optimal. Urine samples are the test of choice for diagnosing chlamydia in men.
Diagnostic tests include urine sample, nucleic acid amplification, culture, and serology. Nucleic acid amplification tests (NAATs) are the most sensitive and can be performed on vaginal or urine specimens (CDC, 2021e).
Women diagnosed with chlamydial infection should be retested approximately three months after treatment (CDC, 2021e). Cultures can be used to diagnose rectal and pharyngeal chlamydia. Chlamydia cultures are also often combined with Pap smears.
TREATMENT OF CHLAMYDIAL INFECTIONS
Oral antibiotics are the standard treatment for chlamydial infections, which can be easily cured (CDC, 2021e). Patients with chlamydial infections should not have sexual intercourse until a week after the beginning of their treatment regimen. Although guidelines exist for the treatment of chlamydia, the healthcare provider must individualize the treatment regimen to meet the specific needs of each individual.
CHLAMYDIA TREATMENT REGIMES
Typical multiple-dose treatment:- Doxycycline 100 mg orally 2x/day for 7 days
- Azithromycin 1 g orally in a single dose
- Levofloxacin 500 mg orally 1x/day for 7 days
For pregnant patients:
Typical treatment:- Azithromycin 1 g orally in a single dose
- Amoxicillin 500 mg orally 3x/day for 7 days
(Workowski et al., 2021)
CASE
Chlamydia
Marie is a 22-year-old college student who seeks care at University Health Services because she has noted a new-onset vaginal discharge. Marie shares with Gail, the women’s health nurse practitioner, that she is in a new relationship and has not used condoms during sexual activity since she is taking birth control pills. Following a discussion about STDs, Marie and Gail agree that a pelvic examination and STD testing are needed. During the examination, the nurse practitioner notes a mucopurulent endocervical discharge and obtains a specimen for nucleic acid amplification testing (NAAT).
Gail explains that she suspects that Marie has chlamydia and that the NAAT will provide a definitive diagnosis. In the meantime, Gail initiates presumptive treatment and writes a prescription for doxycycline 100 mg to be taken twice a day for seven days. Additionally, the nurse practitioner explains the need to abstain from sexual intercourse until Marie has completed the seven days of antibiotics and the vaginal discharge has resolved.
The nurse practitioner also discusses the need for Marie’s sexual partner to receive treatment so that the infection is not passed back and forth. Marie agrees to tell her sexual partner her diagnosis and the need for him to receive treatment.
Gail recommends that Marie receive annual STD testing since she is sexually active and in the high-risk age group for STDs such as chlamydia. Gail also explains the importance of using condoms consistently during sexual intercourse. She further explains that birth control pills prevent pregnancy but do not protect against the transmissions of STIs.
Gail later receives confirmation of the diagnosis of chlamydia and calls Marie to tell her the diagnosis and to reinforce the importance of abstaining from sexual intercourse until symptoms resolve and the course of antibiotics are complete. Marie tells Gail that her partner reports being asymptomatic but has agreed to testing and has made an appointment at the health clinic.
Gonorrhea
Gonorrhea is an infection of the mucous membranes caused by the diplococcus N. gonorrhoeae. It can be transmitted through sexual contact with the penis, vagina, mouth, or anus. It is about one third as common as chlamydial infections, although the two infections frequently coexist in an infected person. Untreated, gonorrhea also increases a person’s risk of acquiring or transmitting HIV. In 2019, approximately 616,392 new cases of gonorrhea were reported in the United States, and it is the second most common notifiable condition. Adolescents and young adults ages 15–24 years are at the highest risk for infection (CDC, 2021h, 2021s).
CLINICAL MANIFESTATIONS
In men, N. gonorrhoeae causes gonorrheal urethritis, producing symptoms such as pain or discomfort on urination and a purulent urethral discharge (CDC, 2021h). Untreated gonorrheal urethritis can develop into epididymitis (Workowski et al., 2021).
In women, N. gonorrhoeae that have invaded the urethral opening produce gonorrheal urethritis, and N. gonorrhoeae that have invaded the vagina produce gonorrheal cervicitis. Most women with urethral or cervical gonorrhea have mild or no symptoms and may infect sexual partners unwittingly (CDC, 2021t). When symptoms do occur, they can include purulent and sometimes odorous vaginal discharge, vaginal bleeding after intercourse, and pain or discomfort on urination. Gonorrhea can also infect the Bartholin’s glands. Untreated gonorrheal cervicitis sometimes ascends into the uterus and leads to PID, which typically presents with lower abdominal pain. On speculum examination, gonorrheal cervicitis appears as a swollen, friable cervix with a mucopurulent exudate.
Untreated, patients with a gonorrheal infection develop gonorrheal bacteremia with fever and, occasionally, septic arthritis. Gonorrheal bacteremia is also known as disseminated gonococcal infection (DGI). DGI is usually characterized by arthritis, tenosynovitis, and dermatitis and can be life threatening (CDC, 2021h). The untreated disease can also cause scarring of the Fallopian tubes, leading to infertility or tubal pregnancies.
It is also common for patients with gonorrhea to have trichomoniasis. In both males and females, oral sex can lead to gonococcal pharyngitis and anal sex to gonococcal proctitis. In the newborn, it manifests as a conjunctivitis that can cause blindness.
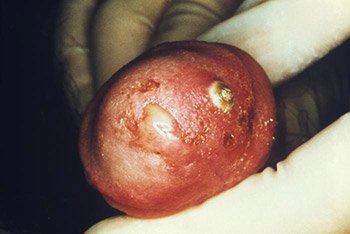
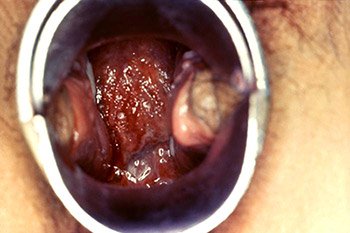
This male presented with a purulent penile discharge and overlying penile pyodermal lesions located on the glans penis, due to gonorrhea. (Source: CDC/Jo Miller, Public Health Image Library.)
This colposcopic view of a patient’s cervix revealed an eroded ostium due to Neisseria gonorrhea infection. (Source: CDC, Public Health Image Library.)
SCREENING AND DIAGNOSIS OF GONORRHEA
Gonorrhea infections are difficult to distinguish clinically, and lab tests are needed to make a definitive diagnosis.
It is recommended that two subpopulations have regular gonorrheal screenings and be counseled about the importance of using condoms:
- Sexually active women younger than 25 years of age
- Sexually active women age 25 and older who have a new sex partner, multiple partners, a partner who has other partners, or a sex partner who is a sex worker or who has an STI
- All pregnant women younger than 25 years of age
- Pregnant women 25 years of age and older who have a new sex partner, multiple partners, a partner who has other partners, or a sex partner who is a sex worker or who has an STI (CDC, 2021i)
- Men who have sex with men, who should receive screening for rectal, urethral, and pharyngeal gonorrhea
(CDC, 2021i; CDC, 2021u)
People younger than 25 years, including sexually active adolescents, are at risk for gonorrhea infection, but there are no recommendations for screening due to insufficient evidence of its effectiveness in that age group.
Light microscopic examination of a sample of urethral discharge can diagnose gonorrhea in men. In male gonorrheal urethritis, stained microscope slides will show Gram-negative diplococci. For women, a more sensitive and specific test is needed, and NAATs of urine or of a swab of the affected area are the preferred test techniques (CDC, 2021h). Urine samples can be used to test for urethritis in all genders.
Vaginal swab specimens are used to test for cervicitis. Gonorrheal cervicitis produces sufficient discharge that swabs need not be taken by speculum examination. Instead, they can be collected blindly by the patient.
For oral or anal gonorrhea, pharyngeal or rectal swabs are taken and sent to the lab for culturing.
TREATMENT OF GONORRHEA
As with many STIs, the highest rates of successful treatments come when antimicrobial medicines can be taken in single doses administered at the time of diagnosis. Antimicrobial resistance is a growing problem in the treatment of gonorrhea, making eradication of the infection challenging in some individuals (CDC, 2021h). Although guidelines exist for the treatment of gonorrhea, the healthcare provider must individualize the treatment regimen to meet the specific needs of each individual.
GONORRHEA TREATMENT REGIMES
For uncomplicated urogenital or anorectal gonorrhea (including for pregnant patients):
- Ceftriaxone 500 mg intramuscularly (this can be reconstituted in 1% lidocaine to reduce pain in the injection area)
- Ceftriaxone 1 gm for those weighing 150 kg or more
For patients with penicillin or cephalosporin allergies:
- Dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g, or
- Oral ciprofloxacin 500 mg in a single dose, if the person has no symptoms and gyrase A testing can be performed to identify ciprofloxacin susceptibility
For pharyngeal gonorrhea:
- Ceftriaxone 500 mg intramuscularly in a single dose, ceftriaxone 1 gm for those weighing 150 kg or more
(CDC, 2021j)
DRUG RESISTANCE AND TREATMENT CONCERNS
One difficulty in treating gonorrhea is that it has developed resistance to the fluoroquinolone antibiotics used to treat it, leaving the cephalosporin antibiotics as the only recommended treatment. If a cephalosporin-resistant gonorrhea were to emerge, this STI would become very difficult to treat (CDC, 2021v).
Patients with gonorrhea often have other STDs/STIs and are therefore tested for HIV, chlamydia, trichomoniasis, and syphilis. They are also offered hepatitis B vaccination if they have not already been vaccinated.
Patients with uncomplicated urogenital or rectal gonorrhea who receive standard treatment recommendations do not require retesting for infection. Patients with pharyngeal infection are retested in 7–14 days using NAAT or cultures. However, if NAAT testing is positive, a culture should be obtained for antimicrobial susceptibility before receiving retreatment (CDC, 2021j).
Although treatment for gonorrhea is usually successful, patients with gonorrhea may become reinfected. Therefore, as for people with chlamydial infections, patients treated for gonorrhea are advised to return in three months for rescreening.
CASE
Gonorrhea
Rogelio, a 32-year-old male, is being seen by his primary care provider, Tara, for what Rogelio believes is a urinary tract infection due to pain on urination. His history reveals that Rogelio has had one sexual partner for the past two months and that condoms are not consistently used. On physical examination, Tara notes a reddened and swollen scrotum and discharge from the urethra. She obtains a swab of the discharge and performs light microscopic examination, noting Gram-negative diplococci consistent with gonorrhea. She also performs testing for chlamydia, which is negative. Tara then asks Rogelio if his partner has had any symptoms, and he states he is not aware of any.
Since Rogelio weighs 62.6 kg, Tara administers ceftriaxone 150 mg intramuscularly, in a single dose, to treat the gonococcal infection. Tara tells Rogelio that it is important that he notify his sexual partner that she may have gonorrhea and needs to be treated. Rogelio tells Tara he will have a discussion with his partner that evening. Tara also tells Rogelio that he must refrain from sexual intercourse until seven days after treatment of himself and his partner.
Rogelio calls the clinic the next day and reports that his partner has refused to go to the clinic for testing. Tara explains that because they live in a state in which expedited partner therapy is allowed, she can prescribe oral antibiotics for Rogelio’s partner to take at home, although this is not the recommended IM route of antibiotic administration to treat gonorrhea. Tara calls a prescription to the pharmacy for cefixime 800 mg given orally as a single dose. Even though an oral antibiotic is not as effective in the treatment of gonorrhea, it is important that Rogelio’s partner receive treatment. Moreover, for maximum adherence, it is recommended that antibiotics be taken under direct observation of a clinician.
Syphilis
Syphilis is caused by the bacterium T. pallidum. In the United States, 133,945 new cases of syphilis were reported in 2020, with men who have sex with men accounting for 43% of all primary and secondary symphilis cases. Case rates are also increasing among heterosexual men and women in recent years (CDC, 2021k, 2021s).
Syphilis is transmitted by sexual contact. Pregnant women with syphilis can also transmit the disease to their newborn baby at the time of birth. The infant can then develop congenital syphilis, which when untreated can delay development, cause seizures, or even be fatal. Congenital syphilis is now referred to as a mother-to-child transmission and is projected to be eliminated from countries with limited resources by rapid method testing and a single injection of penicillin given antepartum (Fan et al., 2019).
CLINICAL MANIFESTATIONS
A syphilis infection begins locally and slowly spreads systemically. Over time, untreated syphilis will go through stages with different signs and symptoms. It can be fatal if untreated because of potential damage to the brain, heart, or nervous system. A person may come to a clinician initially with primary syphilis (i.e., a local genital infection) or a few months later with secondary syphilis (i.e., a systemic infection). The stages of syphilis infection are as follows:
- Primary syphilis. The hallmark of primary syphilis, a local infection, is the appearance of an ulcer called a chancre. Typically, there is only one chancre, located at the site of infection on the penis, vulva, cervix, perianal region, or oral mucosa. The chancre appears a few weeks after T. pallidum bacteria have invaded the skin. The incubation period is approximately 3 weeks. A syphilis chancre typically has firm, raised edges and a smooth internal base and is painless. Local lymph nodes may be enlarged. If untreated, chancres heal spontaneously in 3–6 weeks, leaving faint scars. Even if the chancre has disappeared, the infected person is very contagious during this phase of the disease (CDC, 2021k).
- Secondary syphilis. When primary syphilis is not treated, the chancre disappears for a few weeks and then reappears as secondary syphilis. Secondary syphilis is a systemic infection with flu-like symptoms (low-grade fever, headache, malaise, generalized lymphadenopathy) and a widespread, symmetrical, non-itchy maculopapular rash, first on the trunk and arms and later the palms and soles. The genital area may also have wart-like papules. During secondary syphilis, a person can develop syphilitic hepatitis or syphilitic glomerulonephritis.
- Latent syphilis. When secondary syphilis is untreated, the symptoms usually fade and the disease becomes quiescent, sometimes for years. This asymptomatic interim stage is called latent syphilis.
- Tertiary (late-stage) syphilis. Although rare, untreated syphilis may reemerge and cause symptomatic damage to a variety of organs. This can occur 10–30 years after the initial infection (CDC, 2021k). This is the final form of the disease, and it produces granulomatous or necrotic lesions that can involve the skin, eyes, central nervous system, heart, aorta, or bones. Today, tertiary syphilis is rare except in patients with a concurrent HIV infection.
SCREENING AND DIAGNOSIS OF SYPHILIS
Routine screening for syphilis is recommended for:
- Patients with any newly diagnosed STD/STI
- Patients at high risk for STDs/STIs
- All pregnant women
The laboratory tests to screen for syphilis include Venereal Disease Research Laboratory (VDRL), rapid plasma reagin (RPR), fluorescent treponemal antibody absorbed (FTA-ABS), and T. pallidum particle agglutination (TPPA). Diagnoses are more likely to be made using blood tests (CDC, 2017l).
The RPR and VDRL are called nontreponemal serologic tests because they are not specific for syphilis. False-positive nontreponemal serologic tests occur in patients with autoimmune disorders such as systemic lupus erythematosus and in a few other special populations. It usually takes at least a week after a patient acquires syphilis for these antibody tests to become positive.
People who have syphilis are more likely to have acquired other STIs. Therefore, patients with syphilis should also be tested for HIV, hepatitis B and C, chlamydia, and gonorrhea.

A primary syphilitic lesion, also known as a chancre, can be seen on the penile corona on the right side of the glans penis. The lesion is accompanied by enlargement of the bilateral inguinal lymph nodes. (Source: CDC/Susan Lindsley, Public Health Image Library.)
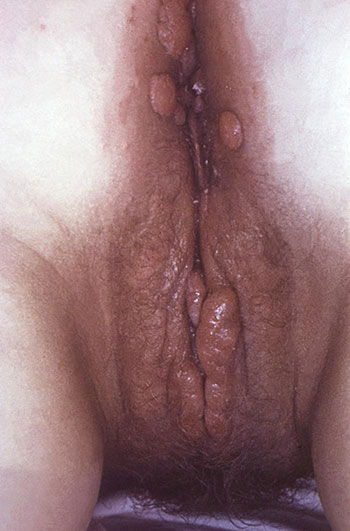
Perianal condylomata lata lesions due to secondary syphilis can be seen in perineal region of a female patient. (Source: Public Health Image Library.)
TREATMENT OF SYPHILIS
Intramuscular benzathine penicillin G is the treatment of choice for syphilis. In the first days after syphilis treatments, some people get a Jarisch-Herxheimer reaction (i.e., fever, myalgia, tachycardia, headaches, and hypotension), informally called Herx, which should not be mistaken for an allergic reaction. Reacting patients are treated with bed rest and nonsteroidal anti-inflammatory agents. Although guidelines exist for the treatment of syphilis, the healthcare provider must individualize the treatment regimen to meet the specific needs of each individual.
SYPHILIS TREATMENT REGIMES
For primary and secondary syphilis in adults:
- Benzathine penicillin G 2.4 million units intramuscularly in a single dose
For latent syphilis in adults:
- Early latent phase: Benzathine penicillin G 2.4 million units intramuscularly in a single dose
- Late latent phase: Benzathine penicillin G 2.4 million units intramuscularly, in 3 doses, at 1-week intervals
For infants and children:
- Benzathine penicillin G 50,000 units/kg (up to 2.4 million units) intramuscularly in a single dose
For patients allergic to penicillin, either oral doxycycline or oral tetracycline is given for 14 days. During pregnancy, however, only penicillin can be given to treat syphilis. Therefore, pregnant women who are allergic to penicillin should first be desensitized to and then treated with penicillin.
(Workowski et al., 2021)
After treatment, patients should be retested at 6, 12, and 24 months for the presence of nontreponemal antibodies. Within a year, the patient’s nontreponemal antibody titers should have decreased at least fourfold. On the other hand, tests for treponemal antibodies will remain positive even after adequate therapy.
For pregnant women with syphilis treated at 24 weeks or earlier, titers should be tested after 8 weeks of treatment and again at delivery. Pregnant women who are treated after 24 weeks of gestation should have titers tested again at delivery (Workowski et al., 2021).
TREATING SEXUAL PARTNERS
Patients with early syphilis (i.e., primary, secondary, or early latent syphilis) are contagious. People who in the preceding three months have had sexual contact with a patient with early syphilis should be notified and treated. Treatment is recommended even for those contacts with no clinical or serologic evidence of the disease (Workowski et al., 2021).
CASE
Syphilis
Marquis, 26-years-old, is seeing his primary care provider, Vedant, because he is concerned about a sore that appeared on his penis one week ago. During the history, Vedant learns that Marquis engages in condomless sex with other men. An examination reveals a chancre with firm raised edges on the underside of the penis, which Marquis reports is painless. Inguinal lymph nodes are enlarged bilaterally.
Vedant suspects that Marquis has primary syphilis and orders VDRL (nontreponemal) and TPPA (treponemal) tests. Since individuals with syphilis are also at risk for other STIs, Vedant explains the need to test for HIV, hepatitis B and C, chlamydia, and gonorrhea.
Vedant explains to Marquis that he is contagious and that anyone with whom he had sexual contact with in the preceding three months must be notified and treated. Vedant explains that the local health department can help Marquis notify his partners of the need for testing and treatment. Marquis declines this service since he feels capable of speaking with his partners about the need for medical care.
Marquis is treated before leaving the office with a single dose benzathine penicillin G 2.4 million units intramuscularly. Vedant explains the need to avoid sexual contact until Marquis’s chancre is healed. Vedant also explains the need to consistently use condoms to avoid the transmission of STIs and that transmission can occur if the sore or lesion is not covered by the condom. Marquis understands that his risks for STIs increases with multiple sex partners.
Marquis verbalizes the need to return for retesting at 2, 12, and 24 months. Vedant explains that within a year, nontreponemal antibodies should be reduced fourfold. If this does not occur, Marquis may need to be treated again. Treponemal antibodies will, however, remain positive.
Genital HPV Infections
HPV infection, caused by small DNA viruses, is the most common STI in the United States. HPV is so common and contagious that almost all sexually active adults will have had at least one genital HPV infection during their lives if they are not vaccinated, and it is estimated that 1 in 100 of every sexually active person has genital warts caused by HPV at any given time. About 14 million people become newly infected each year (CDC, 2020c). About half of the new cases occur in people ages of 15–24. Women are more susceptible to acquiring genital HPV infections than are men. As with other STIs, HPV infections are most common in people with more than one sexual partner (CDC, 2021m; Workowski et al., 2021).
Hundreds of HPV types have been characterized, and approximately 40 of these can infect humans. Benign HPV lesions such as genital warts are caused by low-risk types of HPVs (e.g., HPV types 6 and 11). Rarer, malignant HPV lesions such as cervical cancer and cancers of the anus, penis, vagina, or vulva can be caused or facilitated by high-risk types of HPV (e.g., HPV types 16 and 18).
HPV infections can be sexually transmitted even when an infected person has no visible symptoms, and a condom is used. The common types of genital HPV infections can cause anogenital warts, called condyloma acuminata. HPV types 16 and 18 appear to cause the majority of cervical, vulvar, or penile cancer. HPV infections often disappear spontaneously or may need to be excised (CDC, 2021m; Workowski et al., 2021).
CLINICAL MANIFESTATIONS
Many HPV infections are asymptomatic, but some types of HPV infections cause common skin warts and plantar warts, while other types of HPV infections may cause genital warts and cancer of the vulva, vagina, penis, anus, or back of throat (Mayo Clinic, 2021b; CDC, 2021m).
Genital warts can also develop beyond the genitals. Perianal genital warts can be acquired by skin-to-skin contact, but intra-anal genital warts are transmitted through anal intercourse. Very rarely, newborns can be infected by HPV during the birth process (Syrjänen et al., 2021).
Most HPV infections are short-lived and spontaneously disappear in less than a year without causing clinical problems. However, HPV can cause cancer of the cervix as well as the vulva, vagina, penis, anus, and oropharynx. It can take years to decades for cancer to develop after contracting HPV (CDC, 2021m).
The aberrant cell growth caused by high-risk HPVs leads to squamous cell dysplasias (e.g., cervical dysplasia) and eventually to neoplasms, such as cervical intraepithelial neoplasias, squamous cell cancers, anal squamous cell cancers, or adenocarcinomas. HPV dysplasias in the cervix can usually be detected by Pap tests (NCI, 2021).
Genital warts vary widely in size. They can be as tiny as a pinhead or as large as a small cauliflower (1 cm to 2 cm in diameter), and they can occur singly or in clusters. The small warts tend to be on stalks. Genital warts are typically found on moist surfaces such as the labia, vagina, cervix, urethra, bladder, perianal skin, or anus. In women, the walls of the vagina and the surface of the cervix can also have genital warts (which are often flat patches). When external warts are present, women should have a speculum exam to search for internal warts.
In all genders, genital warts can grow along the internal walls of the urethra or bladder. Large or extensive warts around the urethral meatus indicate that the internal urinary tract should also be examined for warts.
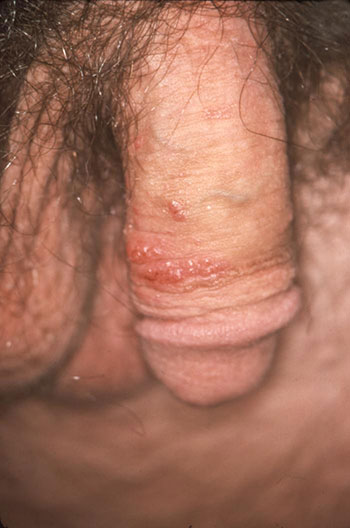
Soft, wart-like growths caused by HPV can be seen on the penile shaft. The areas most commonly affected by genital warts are the penis, vulva, vagina, cervix, perineum, and perianal area. (Source: CDC/Dr. M. F. Rein, Public Health Image Library.)
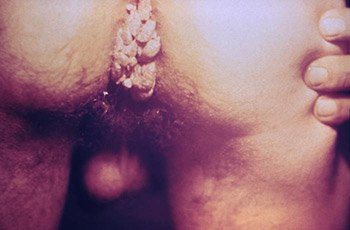
A close view of the anal region of a male patient shows the presence of condylomata acuminata (genital warts). (Source: Public Health Image Library.)
SCREENING AND DIAGNOSIS OF GENITAL HPV INFECTIONS
A variety of organizations suggest that all women ages 21–29 years should have a cytology (Pap) test to screen for cervical cancer every three years. For women ages 30–65 years, a cytology test should be performed every three years, an HPV test every five years, or a cytology and an HPV test (co-testing) every five years. Cervical screening recommendations are the same for women who have received the HPV vaccine (CDC, 2021n).
HPV cannot be cultured. On the other hand, DNA from HPV viruses can be tested to determine whether the virus is one of the high-risk types. DNA testing and type classing are used to make judgments about dysplasias with unclear cytologic test results, and DNA tests are also a common adjunct to Pap testing of women older than 30 years of age. Tissue testing after biopsy provides a definitive diagnosis, as do PCR assays (Harding et al., 2020).
Genital warts, like cutaneous warts, can usually be diagnosed by their appearance, symptoms, and history. Genital warts caused by low-risk HPVs are soft, raised, skin-colored, and nontender. Most genital warts are asymptomatic, although sometimes they cause itching or burning (CDC, 2021m).
HPV VACCINATION
There are three vaccines to prevent HPV licensed in the United States:
- 2vHPV, effective against HPV types 16 and 18
- 4vHPV, effective against HPV types 6, 11, 16, and 18
- 9vHPV, effective against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
As of 2021, the only vaccine available in the United States is 9vHPV. HPV types 16 and 18 are responsible for 66% of cervical cancers, while HPV types 6 and 11 are linked to more than 90% of cases of genital warts.
The CDC’s Advisory Committee on Immunization Practices recommends the following vaccination practices for HPV (Meites et al., 2019):
- Two doses of HPV vaccine in boys and girls ages 9–12 years
- Catch-up vaccination for those up to age 26 years who have not previously received the HPV vaccination
Ideally, the vaccination should be given before a female is sexually active because it does not protect against existing HPV infections. The current HPV vaccine does not protect against all potentially cancer-causing types of HPV. Therefore, all women—even those who have been vaccinated against HPV—should have regular Pap tests.
The vaccine can also prevent genital warts and anal cancers in males and may help to prevent cancers of the oropharynx and penis (ACIP, 2018).
TREATMENT OF GENITAL HPV INFECTIONS
HPV infection itself cannot be treated, but lesions caused by the virus are treated by removal, which reduces infectivity but does not remove the presence of the virus.
About 25% of all genital warts will disappear on their own; nonetheless, most clinicians and patients elect to remove the warts. After treatment, the virus is still in the surrounding epithelium, and approximately one third of patients will have a recurrence of the warts. In addition, HPV infections are commonly reacquired from the patient’s sexual partner(s).
For squamous cell dysplasias, treatment is usually excision. Dysplasias are referred to a specialist for medical management.
GENITAL WARTS TREATMENT REGIMES
For small areas (less than 10 cm2) of genital warts, patients can often treat themselves with topical chemicals. It may be necessary to apply these topical drugs for many weeks. It should be noted that for several hours these chemicals can affect the efficacy of condoms.
Typical patient-applied treatments for external anogenital warts are either:
- Imiquimod 5% cream applied to warts 1x/day at bedtime 3 days per week for less than 16 weeks, or
- Podofilox 0.5% solution or gel applied to warts 2x/day in cycles of 3 days of treatment alternating with 4 days of no treatment, may repeat up to 4 cycles, or
- Sinecatechin 15% ointment applied 3x/day, not to continue beyond 16 weeks
Healthcare provider–applied treatments include:
- Cryotherapy (liquid nitrogen or cryoprobe)
- Chemicals (trichloracetic acid or bichloroacetic acid)
- Surgical excision (tangential scissor excision, shave excision, curettage, laser, or electrosurgery)
For pregnant patients:
- Podofilox, podophyllin, and sinecatechins should be avoided during pregnancy.
- While imiquimod appears to have a low risk (pregnancy category C), it should not be used until more research is obtained.
(Workowski et al., 2021)
CASE
HPV Vaccination
A mother brings her 13-year-old daughter, Rosa, to the pediatrician’s office for her annual well-child check-up. They are seen by Luanne, a pediatric nurse practitioner. Much to Rosa’s embarrassment, her mother starts asking the nurse about “this vaccine for genital warts I’m hearing about on TV. Does Rosa need that if she’s not having sex yet?”
Luanne explains to both Rosa and her mother that the vaccine is a series of injections that protect Rosa from HPV, the virus that causes genital warts. She explains that these warts may cause cervical cancer later in life. The recommendation is that girls receive the immunizations before they are sexually active and can get the warts. Luanne also points out that immunizations are only effective if they are given before exposure to the infection.
The mother thanks Luanne and asks if there is an HPV vaccine for her 11-year-old son. Luanne explains that genital warts in men and boys can become cancerous as well and that the vaccine is also recommended for boys. The mother says she will “think about it and look up some more about it on the Internet.” Luanne offers to answer any questions that may occur later and gives a pamphlet to both Rosa and her mother.
CONTROLLING THE SPREAD OF STIs
The National Strategic plan addresses the prevention of STIs and the prevention and reduction of their adverse effects. Since the majority of reported STDs are diagnosed in physician’s offices and community clinics while patients are receiving care for other conditions, clinicians in these settings must be able to identify and manage STIs/STDs (Barrow et al., 2020).
Preventative strategies to reduce the transmission of STIs include risk assessment and screening, prevention education, barrier protection through the use of condoms, preexposure vaccinations, and expedited partner therapy (HHS, 2021; Workowski et al., 2021).
STI Risk Assessment and Screening
A sexual history should routinely be included when obtaining a medical history. In non-STD specialty settings, clinicians should obtain a sexual history and conduct a risk assessment at an initial visit, annual examination, and any appointment for reproductive, genital, or urologic problems. Physical and pelvic examinations should be performed on any patient with symptoms or concerns related to STDs or in patients with high-risk behaviors for STIs, as well as anoscopy, when indicated (Barrow et al., 2020).
The interview should be conducted in a respectful and nonjudgmental manner. Many infected individuals do not develop or are not aware of symptoms, and thus they do not present themselves to the healthcare system. Other individuals avoid the healthcare system because they are hesitant about acknowledging or reporting symptoms. One method for conducting a sexual history is the use of the “Five Ps” approach (see box below). Risk assessment also includes determining biologic markers through STI and HIV screening tests.
THE FIVE Ps
The CDC recommends that a sexual history include information pertaining to the “Five Ps.” Clinicians can ask patients the following questions:
Partners
- Are you having any type of sex right now?
- What is the gender of each partner?
Practices
- Have you had vaginal sex, meaning “penis in vagina sex”?
- Have you had anal sex, meaning “penis in rectum/anus sex”?
- Have you had oral sex, meaning “mouth on penis/vagina”?
Prevention of Pregnancy
- Would you like to have (additional) children?
- How important is it that you prevent pregnancy now or until you want children?
- Are you or your partner practicing any form of birth control?
- Do you want to discuss pregnancy prevention?
Protection from STDs/STIs
- What do you do to protect yourself from STDs/STIs?
- When do you use a condom?
- Do you have conversations with your partners(s) about preventing STIs and HIV?
- Do you have conversations with your partner(s) about testing for STIs?
Past History of STDs/STIs
- Have you ever had an STD/STI?
- Have any of your partners had an STD/STI?
- Have you ever had an STI test?
- Have you or your partners injected drugs?
- Is there anything else in your sexual practices about which you have questions?
(To determine HIV and viral hepatitis risk:)
(CDC, 2021c)
STI Prevention Education
Preventative education should include at least one STI prevention counseling session of at least 30 minutes (Barrow et al., 2020). Education is tailored to the individual in a culturally sensitive manner. It should also consider gender identity, sexual orientation, age, and the developmental level of the individual (CDC, 2021c).
STI prevention counseling should be provided for all adolescents who are sexually active as well as adults who have an STI, were diagnosed with an STI in the past year, or have multiple sex partners (CDC, 2021c). Education can be one-on-one; in a group format; and use video, motivational interviewing, and online resources. Giving people clear and complete information about STDs/STIs and their prevention has been shown to reduce those people’s risky sexual behaviors and to slow the spread of STIs.
With limited time, money, and personnel, the strategy has been to focus resources on high-risk populations and to emphasize the first-line types of STI protection, such as barrier methods (e.g., condoms). Likewise, expensive screening tests for STIs have been aimed at high-risk populations to get the best results with limited resources.
The clinician’s best opportunity to help prevent the recurrence of an STI/STD is at the time of the patient’s diagnosis. Clinicians explain to the patient that barriers (e.g., condoms) are needed to protect against STIs/STDs and that other birth control methods will not prevent such infections. Patients are also reminded that if their partners are not monogamous, then STIs can enter the relationship between two previously uninfected people (CDC, 2021g).
Healthcare providers must also describe the long-term risks of STIs/STDs. Patients should be told that, although antibiotics will cure them, there is a high risk of reinfection. Therefore, patients are instructed to return to the clinic or office to be retested in 3–4 months.
ADOLESCENT COUNSELING
Primary prevention of STIs/STDs is an important part of adolescent education and healthcare. In a 2019 survey of high school students, 38% reported having had sexual intercourse and 9% having had four or more sexual partners (CDC, 2020e). Of those adolescents who had sexual intercourse in the previous three months, 46% reported not using condoms. Moreover, there is a connection between substance use and high-risk behaviors such as having sex, having multiple sex partners, lack of condom use, and pregnancy before the age of 15 (CDC, 2019).
The CDC (2020e) recommends that STI prevention programs for adolescents:
- Provide basic, accurate information that promotes healthy decisions and behaviors
- Include adolescents who are not having sex in addition to those who are sexually active
- Provide education on how to protect themselves and others against STDs, HIV, and pregnancy
- Involve both adolescents and parents
- Be developed locally to be consistent with community values and policies
Since adolescents who feel a sense of connectedness with important people in their lives are less likely to engage in high-risk sexual behaviors, clinicians should use appointments to establish connections with teens. Adolescents should have the opportunity for private conversations with their healthcare providers during well-visits (CDC, 2020b). Clinicians should discuss sexual behaviors that increase the risk for STIs, including abstinence, the proper and consistent use of condoms, and reducing the number of sexual partners (Workowski et al., 2021).
Barriers As Protection
Condoms reduce the transmission and acquisition of STIs during sexual contact when they are used consistently and worn properly (CDC, 2021p). Patients should be instructed that there is risk of transmission if an area infected by an STI is not covered by the condom.
In theory, female condoms cover more areas of contact during sexual activity than do male condoms, but neither type of condom prevents all skin-to-skin contact. Condoms are more effective in preventing those STIs that are transmitted via fluids (e.g., chlamydia, gonorrhea, HIV) than STIs that are transmitted via direct skin contact (e.g., chancroid, genital herpes, genital warts, syphilis) (CDC, 2021p).
Oral sex is a widespread form of sexual activity. It has been found that 85% of 18- to 45-year-olds and 33% of 15- to 17-year-olds profess to having had oral sex at least once. Chlamydia, gonorrhea, syphilis, HIV, HPV, and trichomoniasis can all be spread by oral sex. Oral sex has a much lower risk for transmission of HIV, but HIV can be spread orally in the absence of protection (CDC, 2017n). Dental dams (latex or polyurethane sheets placed between the mouth and vagina or anus during oral sex) can also reduce the risk of STI transmission.
To be most protective, condoms must be used correctly and all the time. Healthcare workers should not assume that patients know how to use condoms and should demonstrate how to put a condom on a penis or in a vagina using an anatomically correct model. If lubrication is used in conjunction with condoms, patients should be instructed to use only water-based lubrication, as oil-based may cause erosion of the condom.
Preexposure Vaccinations
The major preemptive control of the spread of STIs depends on the development and the use of vaccines that can immunize people against the acquisition of STIs. Public health officials hope that preexposure vaccinations can similarly slow the spread of STIs, although only a few such vaccines are currently available, such as for hepatitis A, hepatitis B, and HPV.
(See also “HPV Vaccination” earlier in this course.)
REDUCING VACCINE DISPARITIES AMONG YOUTH
HPV vaccination can be paid for by the U.S. Vaccines for Children Program. This federal program covers the cost of recommended vaccinations for children through the age of 18 years if they are Medicaid-eligible, uninsured, underinsured for vaccinations, Native American, or Alaskan Native (CDC, 2020c).
Expedited Partner Therapy
With the cooperation of their patients, clinicians should try to find and treat sexual contacts who may have an STD/STI. Often the partner of those with STIs may be asymptomatic and unaware of having an STI or may be unwilling or unable to pursue testing and treatment. Ideally, these partners should be tested for STIs/STDs and then treated in person.
Patients should be asked to notify their sexual partners and to encourage the partners to seek medical care. Some local health departments have programs to help patients notify their partners and to arrange confidential treatment and counseling. The aim of these partner services is to provide clinical and epidemiological services to prevent transmission and reduce complications for people with STDs, partners of people with STDs, and those who are at risk for STDs (CDC, 2020d).
In many states, people who have been diagnosed with chlamydia or gonorrhea may be able to receive medication or a prescription for medication to treat their sex partners, without the partner being seen by a healthcare provider (CDC, 2021o; Jamison & Chang, 2021). This allows the partner to receive rapid treatment, reduces the risk of reinfection by the partner, and decreases the risk of passing the infection to others. According to the CDC, as of April 2021, such “expedited partner therapy” (EPT) is allowable in 46 states and the District of Columbia and potentially allowable in an additional four states and Puerto Rico (CDC, 2021q).
One issue with EPT, however, is that the partner must be treated with oral medications and current guidelines call for intramuscular antibiotic injection for syphilis and gonorrhea. As a result, providers should highly encourage the partners to come to the clinic or provider’s office for treatment (CDC, 2021c).
DISEASE INTERVENTION SPECIALISTS
Some public health departments employ disease intervention specialists (DISs), especially in communities that serve priority populations (HHS, 2020). A DIS helps to reduce STI transmission through interventions, community assessments, education, counseling, and partner services (e.g., contact tracing, collecting lab specimens, and help with navigating the process of evaluation and treatment).
IMPACT OF COVID-19 ON STIs
The COVID pandemic has impacted prevention, detection, and treatment of STIs, in particular, chlamydia, gonorrhea, and syphilis, which were themselves already at epidemic levels. At the start of the COVID-19 pandemic, numerous providers cancelled nonessential in-person appointments at STI clinics, many of which provide services to high-risk and vulnerable populations, such as those without medical insurance as well as priority populations (Napoleon et al., 2020). Due to limited availability of STI testing, the actual impact of the COVID pandemic on STIs is hard to assess (Jamison & Chang, 2021).
In a survey at the start of the pandemic, the National Coalition of STD Directors found that STD services and field visits were postponed in 83% of STD clinics, and 66% of programs reported reduced screening and testing for STIs/STDs. Moreover, 57% of DISs responded that they were reassigned from STI/STD work to COVID-19 contact tracing (NCSD, 2020).
Reported cases of STDs during March and April 2020 were considerably reduced, with reported cases of chlamydia dropping to 50%, gonorrhea to 71%, and syphilis to 64% of cases compared to 2019 levels (CDC, 2021q). Three conditions that were most likely responsible for this decrease in early 2020 included reduced screening for STIs, moving STI/STD staff to COVID-19 responsibilities, and the orders to shelter in place. By the end of 2020, however, the number of reported cases of syphilis and gonorrhea once again rose (see graphic).
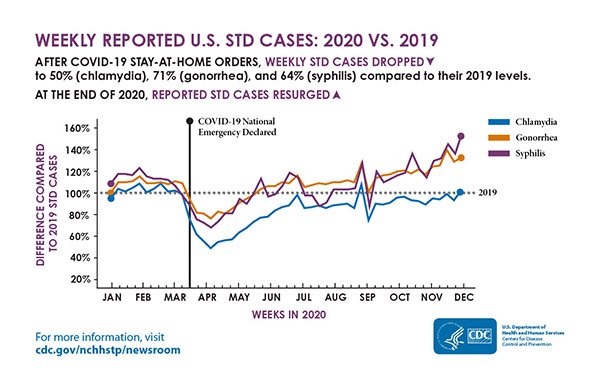
Weekly reported U.S. STD cases: 2020 vs. 2019. (Source: CDC.)
Testing for STIs has been greatly impacted by the COVID pandemic due to limited appointments, reduced hours, staffing issues, loss of finances, or closures. Some clinics moved to virtual appointments or telehealth and treated patients based on symptoms and without testing, leading providers to prescribe oral therapies, rather than intramuscular one-time treatments (Nagendra et al., 2020).
Moreover, the reagents used for STI testing of urine were being used for COVID testing, reducing the availability of this simple test leading to the need to obtain the less desirable urogenital swabs. While the patient can perform the self-swabbing, it must be done at a provider office or laboratory (Jamison & Chang, 2021). When testing is reduced, STIs go untreated.
The COVID-19 epidemic has highlighted the need for new methods of meeting the STI epidemic, including the use of telehealth, the need for self- or home-specimen collection, an increase in the use of EPT, the importance of DIS workers, and the need for other types of nontraditional treatment facilities, such as pharmacies and medical clinics in retail facilities (HHS, 2020).
SEXUAL TRANSMISSION AND COVID-19
COVID-19 is not an STI, but it can be transmitted by contact with saliva and droplets from the mouth and nose of an infected person who is in close contact. Thus far, there has been no evidence of sexual transmission of SARS-CoV-2, although the virus responsible for COVID-19 has been identified in semen and feces of infected people (NCSD and NASTAD, 2020; Tur-Kaspa et al., 2021).
CONCLUSION
The STI National Strategic Plan was developed in response to a recent precipitous rise in STIs and focuses on the four STIs that most significantly affect the health of Americans: syphilis, gonorrhea, chlamydia, and HPV. The National Strategic Plan provides goals and interventions to prevent STIs and successfully treat individuals with STIs and STDs to avoid the long-term health consequences of untreated or inadequately treated STIs/STDs. The Nation Strategic plan recognizes that some populations are more adversely affected by STIs, such as adolescents and young adults, men who have sex with men, pregnant women, and certain racial and ethnic minorities.
Patients at risk for STIs or with asymptomatic or symptomatic STDs may be encountered by clinicians in all healthcare settings. This is especially true since rates of certain STDs have reached epidemic levels. STIs can occur in any individual, regardless of their age, gender identity, sexual orientation, race, or socioeconomic status. So, clinicians must be alert to the possibility of a patient presenting with an STI during a routine health visit or while being seen for another health-related concern.
Clinicians in all healthcare settings must be able to perform a sexual history screening and test for STIs, recognize signs and symptoms of STIs, provide treatment, administer HPV vaccination to those who qualify, and educate about preventing exposure to and transmission of STIs. Education should be individualized and may include the consistent use of barrier protection, pre-exposure vaccinations, and being in a monogamous relationship with a partner who has been tested for STIs. Referral to a specialized STD clinic should be made if a healthcare setting is not able to perform these functions or provide for EPT. STD clinics may have specialized DIS staff to assist with partner services.
The COVID-19 pandemic has intensified the challenges of educating, screening for, and treating STIs and STDs. With the onset of the pandemic, resources for detection and treatment of STIs were diverted to address the COVID-19 pandemic.
RESOURCES
HPV vaccination & cancer prevention (CDC)
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Sexually transmitted diseases (NIH, MedlinePlus)
Sexually transmitted diseases treatment guidelines (CDC)
Sexually transmitted infections (WHO)
STI (Teensource)
Teacher’s guide: STDs (KidsHealth)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Advisory Committee on Immunization Practices (ACIP). (2018). Humanpapilloma virus (HPV). Retrieved from http://www.immunize.org
Barrow RY, Ahmed F, Bolan GA, & Workowski KA. (2020). Recommendations for providing quality sexually transmitted diseases clinical services. MMWR, 68(5), 1–19. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021a). HPV infection. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021b). Adolescents and young adults. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021c). Sexually transmitted infections treatment guidelines, 2021. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021d). Chlamydia statistics. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021e). Chlamydia—CDC fact sheet (detailed version). Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021f). Which STD tests should I get? Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021g). Condoms and STDs: fact sheet for public health personnel. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021h). Gonorrhea—CDC fact sheet (detailed version). Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021i). Screening recommendations and considerations referenced in treatment guidelines and original sources. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021j). Gonococcal infections among adolescents and adults. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021k). Syphilis—CDC fact sheet (detailed version). Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021l). CDC estimates 1 in 5 people in the U.S. have a sexually transmitted infection. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021m). Human papillomavirus (HPV): HPV fact sheet. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021n). Sexually transmitted infections treatment guidelines, 2021: HPV-associated cancers and precancers. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021o). Sexually transmitted diseases: expedited partner therapy: an important STD prevention tool. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021p). Condoms and STDs. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021q). Trends in STD case reports during the U.S. COVID-19 pandemic, January–December 2020. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021r). Lesbian, gay, bisexual, and transgender health: transgender persons. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021s). National overview of STDs, 2019. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021t). Sexually transmitted diseases (STDs): STDs & infertility. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021u). Sexually transmitted infections treatment guidelines, 2021: MSM. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021v). Gonorrhea: antibiotic resistance. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020a). STD testing: information for parents of adolescents. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020b). Adolescent connectedness. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020c). Vaccines and preventable diseases: paying for HPV vaccine. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020d). Sexually transmitted diseases: partner services. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2020e). Adolescent and school health. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2019). Adolescent and school health: Substance use and sexual risk behaviors among youth. Retrieved from https://www.cdc.gov
Fakile Y, Brinson M, Mobley V, et al. (2019). Performance of the syphilis health check in clinic and laboratory-based settings. Sexually Transmitted Diseases, 46(4), 250–3.
Fan SR, Wang AL, & Wang LH. (2019). Elimination of mother-to-child transmission of syphilis: challenge and solution. Maternal-Fetal Medicine, 1(2), 95–104.
Harding MM, Kwong J, Roberts D, Hagler D, & Reinisch C. (2020). Lewis’s medical-surgical nursing: assessment and management of clinical problems (11th edition). St. Louis: Elsevier.
Jamison CD & Chang T. (2021). COVID-19 has made it harder to slow the rise in sexually transmitted infections. Expedited partner therapy can help. Health Affairs Blog. Retrieved from https://www.healthaffairs.org
Mayo Clinic. (2021a). Sexually transmitted diseases. Retrieved from https://www.mayoclinic.org
Mayo Clinic. (2021b). HPV infection. Retrieved from https://www.mayoclinic.org
Meites E, Szilagyi PG, Chesson HW, et al. (2019). Human papillomavirus vaccinations for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR, 68(32), 698–702.
Nagendra G, Carnevale C, Neu N, Cohall A, & Zucker J. (2020). The potential impact and availability of sexual health services during the COVID-19 pandemic. Sexually Transmitted Diseases, 47(7), 434–6.
Napoleon SC, Maynard MA, Almonte A, et al. (2020). Considerations for STI clinics during the COVID-19 pandemic. Sexually Transmitted Diseases, 47(7), 431–3.
National Cancer Institute (NCI). (2021). HPV and cancer. Retrieved from https://www.cancer.gov
National Coalition of STD Directors (NCSD). (2020). COVID-19 & the state of the STD field. Retrieved from https://www.ncsddc.org
National Coalition of STD Directors (NCSD) and NASTAD. (2020). Sex and Covid-19: FAQs. Retrieved from https://npin.cdc.gov
Ness SM. (2020). LGBTQ cultural competence training. Wild Iris Medical Education. Retrieved from https://wildirismedicaleducation.com
Syrjänen S, Rintala M, Sarkola M, Willberg J, Rautava J, Koskimaa H, et al. (2021). Oral human papillomavirus infection in children during the first 6 years of life, Finland. Emerging Infectious Diseases, 27(3), 759–66.
Tur-Kaspa I, Tur-Kaspa T, Hildebrand G, et al. (2021). COVID-19 may affect male fertility but is not sexually transmitted: a systematic review. Fertil Steril Rev, 2(2), 140–9.
U.S. Department of Health and Human Services (HHS). (2020). Sexually transmitted infections national strategic plan for the United States: 2021–2025. Washington, DC: author. Retrieved from https://www.hhs.gov
U.S. Department of Health and Human Services (HHS). (2019). STI national strategic plan overview. Retrieved from https://www.hhs.gov
Workowski KA, Bachmann LH, Chan PA, et al. (2021). Sexually transmitted infections treatment guidelines, 2021. MMWR Recommendations and Reports, 70(4), 1–192.



Customer Rating
4.9 / 409 ratings