Pain Management for Oregon Nurses and Other Healthcare Professionals
Online Continuing Education Course

Course Description
MANDATORY OREGON PAIN MANAGEMENT CEU FOR NURSES AND OTHER HEALTHCARE PROFESSIONALS. This course fulfills 6 of the 7 hours of pain management CE required of many licensed healthcare professionals in Oregon (the additional 1 hour must be taken from the Oregon Pain Management Commission). Covers pain assessment, interventions, and self-management strategies; roles and guidelines for nursing, occupational therapy, and physical therapy; issues regarding opioid use.
Course Price: $60.00
Contact Hours: 6
Pharmacotherapeutic Hours: 1.25
Course updated on
January 13, 2023
"Very comprehensive. I know I will refer back to the material in the future. I felt it promoted a compassionate approach to this difficult topic." - Michele, RN in Oregon
"I loved this course and learned so much. I have been a hospice and home health nurse for over 30 years and wish I had this information all those years." - Brenda, RN in Oregon
"I found the information thorough, comprehensive, useful, and up-to-date!" - Sabrina, RN in Oregon
"Well done. I appreciate the well-developed content." - Timothy, RN in Orgeon
Pain Management for Oregon Nurses and Other Healthcare Professionals
Copyright © 2023 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will better understand the experience of pain, appropriate assessment and interventions for pain, and issues regarding opioid use. Specific learning objectives to address potential knowledge gaps include:
- Explain the experience and physiology of pain.
- Outline the elements of a comprehensive pain assessment.
- Describe pharmacologic and nonpharmacologic interventions and self-management strategies.
- Discuss the roles and guidelines for prescribers, nursing, occupational therapy, and physical therapy in pain management.
- Discuss the issues of opioid misuse, abuse, and diversion and drug-seeking behaviors.
TABLE OF CONTENTS
Introduction
The word pain comes from the Greek (poiné) and Latin (poena) words for punishment or penalty. In the time of Aristotle and other Greek philosophers, pain was believed to be visited on a person from external sources, in particular, the gods. During the Renaissance, pain was believed to arise from an internal mechanical process, and this theory of pain persisted well into the twentieth century.
Modern pain research began in the 1960s, and in recent decades, there has been a change in the perception of pain that has profoundly influenced scientific and medical pain research and treatment. Pain is no longer viewed as a symptom but rather a disease in and of itself. Its occurrence, severity, duration, response to treatment, and disabling consequences vary from person to person. Like other diseases, pain is more than a biological phenomenon; it has profound emotional and cognitive effects.
In 2010, the Council of the International Association for the Study of Pain (IASP) issued the Declaration of Montreal, which asserts that “withholding of pain treatment is profoundly wrong, leading to unnecessary suffering which is harmful.” The Declaration further asserts:
- Article 1. The right of all people to have access to pain management without discrimination
- Article 2. The right of people in pain to acknowledgment of their pain and to be informed about how it can be assessed and managed
- Article 3. The right of all people with pain to have access to appropriate assessment and treatment of the pain by adequately trained healthcare professionals
(IASP, 2021a)
To meet this obligation, effective management of pain requires an in-depth knowledge of the complexity of the pain experience, enhanced assessment skills, treatment modalities currently available, and policies that affect how these modalities may be utilized.
THE EXPERIENCE OF PAIN
Pain is the single most common reason people seek medical care (NCCIH, 2022). It is a complex experience that includes a physiologic and a psychological response to noxious stimuli. It is a warning mechanism protecting an individual by influencing them to withdraw from harmful stimuli and is primarily associated with injury or the threat of injury.
Pain is subjective and difficult to quantify, as it has both affective and sensory components. The neuroanatomic basis of pain reception develops before birth, and individual pain responses are learned in early childhood. These responses are affected by social, cultural, psychological, cognitive, and genetic factors (Meldrum, 2021).
What Is Pain?
In 1979 the International Association for the Study of Pain defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” The newer 2020 definition (below) replaces terminology that relied upon a person’s ability to describe the experience to qualify as pain. Unlike the older definition, the newer definition no longer excludes infants, elderly people, and others—even animals—who cannot verbally articulate their pain.
- Pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors.
- Pain and the detection of painful stimuli are different phenomena. Pain cannot be inferred solely from activity in sensory neurons.
- Through their life experiences, individuals learn the concept of pain.
- A person’s report of an experience as pain should be respected.
- Although pain usually serves an adaptive role, it may have adverse effects on function and social and psychological well-being.
- Verbal description is only one of several behaviors to express pain; inability to communicate does not negate the possibility that a human or a nonhuman animal experiences pain.
(IASP, 2021a)
Pain alters the quality of life more than any other health-related problem. It interferes with sleep, mobility, nutrition, thought, sexual activity, emotional well-being, creativity, and self-actualization. Surprisingly, even though pain is such an important obstacle to comfort, it is one of the least understood, most undertreated, and oft-discounted problems of healthcare providers and their patients.
CONSEQUENCES OF UNTREATED/UNDERTREATED PAIN
Chronic, persistent untreated or undertreated pain prolongs systemic and chemical brain changes leading to psychological changes. Over time, these can impact brain function, resulting in changes in behavior. Chronic pain and the resulting prolonged stress response can raise blood pressure, increase respiratory and heart rates, and cause muscle tension, all of which can lead to fatigue, sleeping problems, and changes in appetite.
Activation of complex brain systems as a result of chronic pain may increase the person’s awareness of pain and decrease pain tolerance. Evidence also supports the idea that persistent pain can result in changes in the brain that control cognitive function.
Pain, and the fear of pain, can cause avoidance of both social and physical activities. Chronic pain can limit everyday activities and can affect involvement with friends and family, resulting in feelings of isolation.
Pain can cause depression, very common among those with chronic pain, or make existing depression worse; vice versa, depression can also make existing pain worse.
Research shows that uncontrolled pain has an adverse effect on the immune system and lowers the body’s ability to respond to stressful situations.
Consequences of untreated or undertreated pain can also result from pain due to damage to a nerve. This type of pain causes changes in the nervous system that contribute to the development of chronic pain long after the damage to the nerve has healed (NLM, 2021; Padgett, 2019).
| Term | Definition |
|---|---|
| (IASP, 2021a) | |
| Allodynia | Pain due to a stimulus that does not normally provoke pain |
| Analgesia | Absence of pain in response to stimulation that would normally be painful |
| Causalgia | A syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion |
| Dysesthesia | An unpleasant abnormal sensation, whether spontaneous or evoked |
| Hyperalgesia | Increased pain from a stimulus that normally provokes pain |
| Hyperpathia | A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold |
| Hypoalgesia | Diminished pain in response to a normally painful stimulus |
| Neuralgia | Pain in the distribution of a nerve or nerves |
| Neuropathic pain | Pain caused by a lesion or disease of the somatosensory nervous system |
| Nociception pain | Pain arising from actual or threatened damage to non-neural tissue due to the activation of nociceptors (high-threshold sensory receptors of the peripheral somatosensory nervous system) |
| Pain threshold | Amount of pain required before individuals feel the pain; the lower the threshold, the less pain can be endured; the higher the threshold, the more pain can be endured |
| Pain tolerance level | Maximum intensity of a pain-producing stimulus that a subject is willing to accept in a given situation; the subjective experience of the individual |
| Paresthesia | An abnormal sensation whether spontaneous or evoked |
Classification of Pain
The classification of pain is complicated, and there are several different classification systems, many of which overlap. Among other characteristics, pain can be classified by duration and source.
BY DURATION
Pain is classified by duration as acute or chronic.
Acute Pain
Acute pain is protective in that it motivates a person to take action immediately. Acute pain is caused by noxious stimulation due to injury, a disease process, or the abnormal function of muscle or viscera. Acute pain begins suddenly, is usually sharp in quality, and correlates with the amount of damage. It is temporary and subsides as healing takes place. In acute pain, the central nervous system is intact, and acute pain is a symptom. Examples of causes of acute pain include:
- Surgery
- Broken bones
- Dental work
- Burns or cuts
- Labor and childbirth
There are two types of acute pain:
- Somatic pain results from superficial injury to skin and subcutaneous tissue (e.g., burns, cut, abrasions) or deep injury to muscle, bone, joint, and connective tissues (e.g., fractures, arthritis, fibrositis, rupture of muscle belly).
- Visceral pain results from injury to the internal organs (e.g., peptic ulcer, angina pectoris, renal colic).
In most instances, acute pain does not last longer than six months and disappears when the underlying cause of pain has been treated or has healed. Severe acute pain activates the sympathetic nervous system, causing diaphoresis, increased respiratory and pulse rates, and elevated blood pressure. Psychological effects of unrelieved pain can lead to anxiety and depression, and unrelieved acute pain may lead to chronic pain (Cleveland Clinic, 2022).
Chronic Pain
Chronic pain is ongoing and usually lasts longer than six months. This type of pain continues even after the injury or illness that caused it has healed. Chronic pain persists, recurs, or progresses over a long period of time and is often resistant to medical treatments. Pain signals remain active in the nervous system for weeks, months, or years. Some people suffer chronic pain even when there is no past injury or apparent body damage. Chronic pain is linked to such conditions as:
- Headache
- Arthritis and other musculoskeletal conditions
- Cancer
- Chemotherapy/radiation
- Nerve pain
- Back pain
- Fibromyalgia
- Surgical complications
Because the central nervous system may be dysfunctional, chronic pain may be considered a disease state. Chronic pain serves no biologic purpose and has no obvious end-point. If pain is associated with a disease or injury, it outlasts the normal period of healing, and the severity does not correlate with damage.
With chronic pain, stress affects the body, producing physical conditions such as tense muscles, limited ability to move about, lack of energy, and changes in appetite. Chronic pain also causes emotional effects that may include depression, anger, anxiety, or fear of reinjury (Cleveland Clinic, 2022).
BY SOURCE
The sources (causes) of pain are divided into the categories of nociceptor, neuropathic, psychogenic, and idiopathic.
Nociceptor Pain
Nociceptor pain is acute pain that results when tissue damage produces a stimulus that sends an electrical impulse across a receptor (nociceptor) by way of a nerve fiber to the central nervous system. Receptors for this type of pain are located all around the body, particularly under the skin and the internal organs. Some body tissues, such as the brain and lung, have no nociceptors, and some tissues have many.
Nociceptive pain can be divided into those that are sustained by injury to somatic tissues (bone, joints, or muscles) and those that are sustained by injury to visceral tissues. Somatic pain is often described as aching, stabbing, throbbing, or pressure-like. Visceral pain is usually described as gnawing or crampy when arising from obstruction of a hollow organ such as the bowel, and as aching or stabbing when arising from other internal organs.
Nociceptor pain is:
- Well-localized
- Worse with movement
- The result of obvious tissue injury or illness
- Caused by inflammation
- Physiologic
(Portenoy &Dhingra, 2020)
Nociplastic Pain
Nociplastic pain arises from altered nociception despite the absence of clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system. This type of pain may reflect changes in the way the nervous and immune systems function (Slater & Davies, 2021).
Neuropathic Pain
Neuropathic pain results from damage to or dysfunction of the peripheral or central nervous system rather than from stimulation of pain receptors. Mechanisms of neuropathic pain are complex and involve changes:
- At the peripheral nociceptor and nerve level
- At the dorsal root ganglion (DRG)
- In the central nervous system, nociceptive pathways, and terminal structures
Changes at the peripheral nociceptor and nerve level reduce the threshold for activation and increase the response to noxious stimuli. In chronic states, the peripheral nerve continuously triggers signals to the central nervous system.
Neurons located within the dorsal root ganglion are responsible for sensory transduction and modulation from the periphery, including pain perception. DRGs are involved in the process of chronicity, from acute to chronic pain, even after resolution of the original insult.
In the central nervous system (CNS), pain is caused by dysfunction of somatosensory pathways in the CNS. Central neuropathic pain develops only due to malfunction of the spinothalamic tract within the spinal cord, which is responsible for crude touch, pressure, and temperature.
One example of neuropathic pain is phantom limb syndrome, which can occur following an amputation and when the brain continues to receive pain messages that originally carried impulses from the missing limb (Watson, 2022).
Neuropathic pain caused by lesion or disease in the peripheral nerves may be due to:
- Traumatic brachial plexus injury
- Diabetes mellitus
- Carpal tunnel syndrome
- Postherpetic neuralgia
Neuropathic pain caused by a lesion or disease of the central nervous system may be due to:
- Central poststroke pain
- Spinal cord injury
Neuropathic pain:
- Is not well-localized
- May be burning or shooting
- May be a feeling of numbness
- Can be experienced as “pins and needles”
- May be due to tissue injury that is not evident
(IASP, 2021b)
Radicular Pain
Radicular pain is a very specific type of pain that can occur when the spinal nerve becomes compressed or inflamed. It radiates from the back and hip to the leg(s) by way of the spine and spinal nerve root. People with radicular pain may experience tingling, numbness, and muscle weakness (Beaumont Health, 2022).
Psychogenic Pain
Psychogenic pain is believed to be sustained mainly by psychological factors. It does not refer to the common idea that pain experienced by some patients is exacerbated by psychological factors, or the finding of high pain-related distress or comorbid psychiatric disease. Instead, it implies that the pain is best understood as a result of psychological processes. It is classified as a somatic symptom disorder with prominent pain, which is diagnosed on the basis of excessive thoughts, feelings, or behaviors related to pain that are distressing, impair function, and appear out of proportion to physical findings.
It must be remembered that psychogenic pain is truly experienced and is not a deception. This distinguishes it from disorders that reflect a serious mental disorder in which reports of pain may not indicate a true experience of pain, and from malingering (Portenoy & Dhingra, 2020).
Characteristics of psychogenic pain include:
- Nonlocalized pains that encompass large parts of the body
- Constant discomfort despite treatment
- Difficulty describing location, quality, and depth of pain
- Worsening pain independent of any underlying medical condition
Idiopathic Pain
Idiopathic pain, also called pain of unknown origin, is chronic pain lasting six months or longer that has no identifiable cause. Although its origin is often unknown, idiopathic pain is very real. It is also possible for this type of pain to remain long after a medical condition has healed when pain normally should have ended. Conditions in which the origin of pain may either be known or be idiopathic include:
- Fibromyalgia syndrome
- Multiple sclerosis
- “Ice-pick” headaches (pain in optic nerves)
- Irritable bowel syndrome (IBS)
- Temporomandibular joint disorder (TMJD)
(Jacques, 2021)
Physiology of Pain
Pain occurs when a noxious signal sends impulses to the spinal cord, which relays it to the brain, where it is interpreted as pain and localized. The brain determines the meaning of the signal and what should be done about it and then sends back instructions to the body about how to respond. This system is the same for everyone, but the sensitivity and efficacy of these brain circuits determines how much the person feels and how the person copes with pain.
In response to a noxious stimulus, an involuntary and nearly instantaneous movement (reflex) occurs. The path taken by the nerve impulses in a reflex is called a reflex arc. Most sensory neurons do not pass directly to the brain but synapse in the spinal cord. This allows physical actions to occur relatively quickly by activating spinal motor neurons without the delay of routing signals through the brain. The brain, however, will receive sensory input while the reflex action occurs (Medicine LibreTexts, 2020).
TISSUE DAMAGE
Receptors (nociceptors) located in the skin and other tissues are nerve fibers with endings that can be excited by three types of stimuli—mechanical, thermal, and chemical. When tissue is damaged, there is an immediate release of inflammatory chemicals (called excitatory neurotransmitters) such as serotonin, histamine, and bradykinin (a powerful vasodilator). Increased blood in the area causes the injured area to swell, redden, and become tender. The bradykinin stimulates the release of prostaglandins and substance P, a potent neurotransmitter that enhances the movement of impulses across nerve synapses (Meldrum, 2021).
MEDIATION
Pain is mediated (caused) by two major types of nociceptor nerve fibers (A-delta fibers and C fibers), which are the nerve endings of the first-order neurons in the pain pathway.
The A-delta fibers are the larger of the two and the most rapidly conducting (12–30 m/sec) because of their thin myelin covering. They respond to thermal, mechanical, and chemical stimuli, and are responsible for the sharp, well-localized pain that is first perceived.
C fibers are smaller, and because they are unmyelinated, impulse signals are slower (0.5 m/sec). C fibers respond to chemical, mechanical, and thermal stimuli, and are associated with the longer-lasting, burning, dull, and poorly localized sensations that follow the first sensation of pain.
Impulse signals travel via the spinal nerves to the spinal cord, where they synapse with second-order neurons in the dorsal horn of the spinal cord. The second-order neurons then cross over to the other side of the spinal cord before ascending to the opposite side of the brain from that part of the body sending the impulse.
Two different pathways—the spinothalamic and spinoreticular tracts—transmit impulses to the brainstem and thalamus. Spinothalamic input is thought to affect the conscious sensation of pain, and the spinoreticular tract is thought to affect the arousal and emotional aspects of pain (Meldrum, 2021).
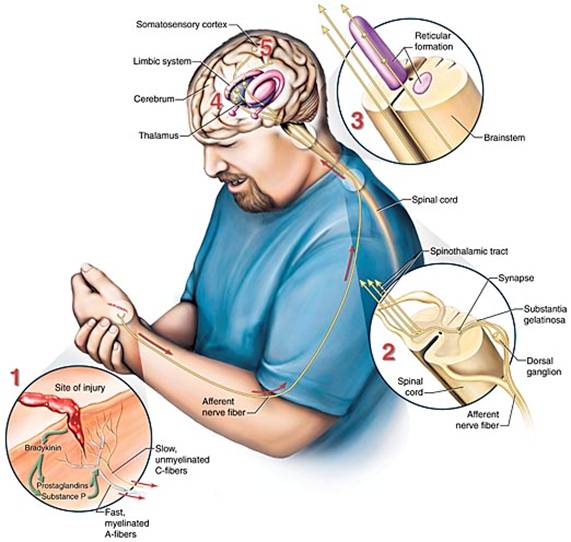
Neurologic transmission of pain stimuli. (Source: Jason M. Alexander. © 2005, Wild Iris Medical Education.)
PERCEPTION
In the cortex of the brain, a sensory strip receives the nerve impulse. This sensory strip, the somatosensory homunculus (Latin for “little man”), is basically a brain map of the body. Nerve impulses travel to the area in the homunculus that corresponds to the part of the body the signal is coming from. All information from each body part (e.g., the finger) end up in one specific area of the homunculus after entering the sensory strip in the cortex of the brain (Kahn Academy, 2022).
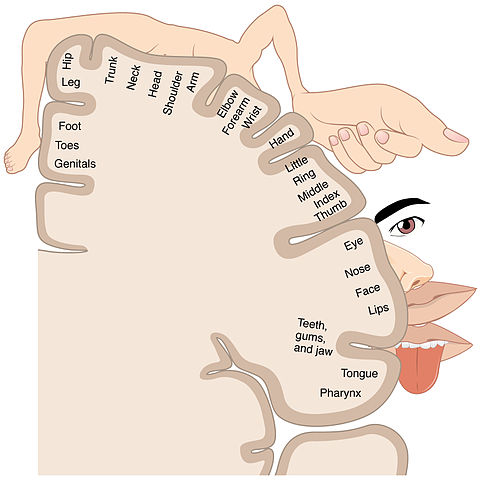
Somatosensory homunculus, or sensory strip. (Source: BodyParts3D, © The Database Center for Life Science.)
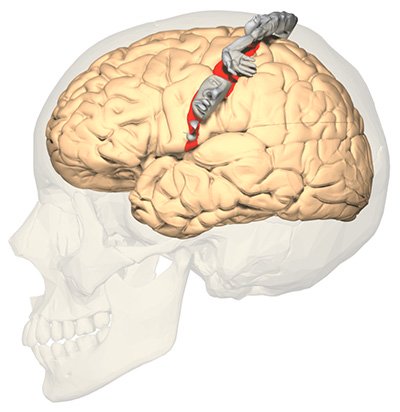
The somatosensory homunculus (“little man”) shows a distorted image of the human body because the size of each region of the map is related to the density of sensory receptors in the body part. (Source: OpenStax College, Anatomy & Physiology.)
Perception of pain results from the brain’s processing of new sensory input with existing memories and emotions. When the message is received in the homunculus, the brain recognizes the pain stimulus and interprets its significance. Several factors can affect how the brain interprets the pain, including:
- Emotional and psychological states
- Memories of previous pain
- Upbringing
- Expectations of and attitudes toward pain
- Beliefs and values
- Age
- Gender
- Social and cultural influences
(Meldrum, 2021)
MODULATION
Pain modulation, the process of alterations in pain signals along the transmission pathway of pain, explains why individuals respond to the same stimulus differently. Once the brain perceives the pain and determines its meaning, it sends messages downward to affect the sensitivity and behavior of nerves. The body releases neuromodulators, including endogenous opioids (endorphins and enkephalins), serotonin, norepinephrine, and gamma aminobutyric acid. These chemicals hinder the transmission of pain and help produce an analgesic, pain-relieving effect.
The descending paths of the efferent fibers extend from the cortex down to the spinal cord and may influence pain impulses at the level of the spinal cord. This system provides a necessary survival function, as it regulates fear and anxiety, allowing the pain experience to be altered according to the situation rather than having pain dominate (Dafny, 2020).
GATE-CONTROL THEORY
In 1965 Melzack & Wall suggested that there is a gate or control system in the dorsal horn of the spinal cord through which all information regarding pain must pass before reaching the brain. An open gate means transmission cells (t-cells) can carry signals to the brain, where pain is perceived. A closed gate stops the transmission, and no pain signal is sent to the brain.
The substantia gelatinosa (SG) in the dorsal horn of the spinal cord controls whether the gate is open or closed. The SG has both excitatory (SG+) and inhibitory (SG-) synapses with the t-cells. There are three kinds of neurons that send signals to the SG. Two of them, A-delta fibers and C fibers, transmit pain signals and open the gate. The third type of neurons, A-beta fibers, responds to nonpainful stimuli such as touch, inhibits the transmission of pain signals, and closes the gate.
An understanding of how this gate-control theory works can be realized by considering a bump to the elbow. When the injury to the elbow occurs, the A-delta fibers are activated, followed by activation of the C fibers, and the pain signal is transmitted. By rubbing the bump, the large, fast- conducting A-beta fibers are stimulated. This stimulation is nonpainful, and the signal is transmitted faster than the A-delta and C fiber signals. The A-beta transmission reaches the SG-, closes the gate, and inhibits the further transmission of pain.
Because the perception of pain has a large cognitive component (e.g., distraction, thoughts, emotions), fast-conducting fibers from the thalamus and cerebral cortex areas of the brain can diminish pain by sending an inhibitory signal through the SG and thus close the gate (Weber, 2022).
Factors That Influence the Experience of Pain
The experience of pain is influenced by both physiologic and psychosocial factors, all of which clinicians must consider in pain management.
PHYSIOLOGIC FACTORS
Physiological factors that influence pain include age, gender, genetic makeup, and stress response.
Age
Multiple studies have demonstrated that age-related changes in peripheral and central nervous systems affect all levels of pain processing. The main findings from studies on pain sensitivity include an increased threshold and decreased tolerance. These changes begin in middle-age, when the prevalence of chronic pain is starting to peak.
As age increases, there is a decrease in somatosensory perception related to the loss of nociceptors and mechanoreceptors and to reduced blood flow to the skin. Neuronal fiber loss and reduced conduction velocity are also associated with reduced sensitivity. Peripheral nociceptors contribute little to the development of chronic pain in the older adult, and this explains why peripherally acting analgesics (such as NSAIDs) can have little effect in an older population. As the individual ages, the processes by which the body alters a pain signal (modulation) as it is transmitted along the pain pathways appears to become less efficient.
There is a lack of evidence that examines chronic pain in children and adolescents, however, available literature suggests that older patients have a higher prevalence of chronic pain than young patients.
At the brain level, despite a significant reduction in grey and white matter typical of the aging process, functional MRI studies reveal that the brain activation in response to painful stimuli and central processing pathways remain unchanged even in extreme age and in moderate cognitive impairment. Significant changes in brain structure have been found in older people with chronic pain, but it is unclear whether these changes are caused by chronic pain or are a predisposing factor to the development of more severe pain perception (Tinnirello et al., 2021; Mills et al., 2019).
Gender
Women appear to suffer pain more often and with greater emotional stress than do men, but some evidence shows that women may cope with severe pain more effectively than men. Men are less likely to report or experience chronic pain than women, and girls are more likely to report having pain in multiple sites than boys.
Studies about how gender (role) and sex (biological) differences are related to the way men and women experience pain have been carried out, which find that women have lower pain thresholds and tolerance and are more likely to experience greater intensity and unpleasantness with pain.
Currently, there is insufficient information about the mechanisms behind these sex-specific differences in pain perception and pain prevalence, but there is some evidence for the role of estrogen and genetics. The fluctuating nature of female hormones may amplify the body’s perception of pain. When estrogen levels are low during the menstrual cycle or after menopause, pain receptor activity is elevated, causing the body to experience more pain.
Studies looking at biological sex have found that at puberty, the rate of pain rises more in girls than boys. And as women age and enter menopause, hormonal levels change and sex differences in chronic pain rates begin to disappear (Dance, 2019).
Genetic Makeup
Studies to date find that there are over 200 genes involved in pain processing and perception. These genes:
- Affect pain independently or jointly, by interaction with environmental factors
- Affect susceptibility to diseases that may cause pain
- Affect susceptibility to more severe and more chronic pain
- Reduce or protect from pain
Genetics can explain heritability in:
- Low back pain (68%)
- Neck pain (58%)
- Migraines (39%–58%)
- Menstrual pain (55%)
- Chronic postsurgical pain (50%)
(Ratka, 2020)
Stress Response
Pain acts as a survival signal for the brain, telling the brain to prepare for “fight or flight.” In response, the brain changes both physically and chemically. This is coupled with changes in the body, such as increased heart rate, prioritization of blood flow to the muscles, and other stress responses, including neural, endocrine, and behavioral changes. Some people are more sensitive and react to this stress, while others are more resilient. Normally, the body resolves these changes and returns to normal following temporary pain. Chronic pain, however, presents different problems.
Chronic persistent pain prolongs the systemic and chemical brain changes, which in turn leads to psychological changes. Over time, these changes can impact brain function, resulting in changes in behavior. This chronic stress, however, is not limited to psychological effects alone. Chronic pain and the resulting prolonged stress response can lead to cardiac issues and gastrointestinal changes, among other things (Padgett, 2019).
PSYCHOSOCIAL FACTORS
Pain perception is the result of the brain’s processing of new sensory input with existing memories and emotions. Childhood experiences, cultural attitudes, heredity, and gender contribute to the development of pain perception and response to pain. Some people may be physiologically able to withstand pain better than others, and cultural factors rather than heredity usually account for that ability.
Depression and anxiety can lower pain thresholds. Anger and excitement can obscure or lessen pain temporarily. Feelings of emotional relief can also lessen a painful sensation (Meldrum, 2021).
Personality
Conflicting evidence exists regarding the role of personality on the variability of pain perception. There are numerous studies, however, that show relationships between sensitivity to pain and the personality traits of neuroticism, extraversion, and openness to experience (see below). Individuals who are more sensitive to pain tend to be high in neuroticism while low in openness to experience and extraversion The personality trait of neuroticism is considered to be among the most significant moderators of pain.
- Neuroticism, a negative personality trait, is characterized by sadness, moodiness, and emotional instability. Individuals with neuroticism exhibit a short pain tolerance and a high pain intensity.
- Openness to experience, a positive personality trait, is characterized by being more willing to embrace new things, fresh ideas, and novel experiences. Persons with this personality trait engage in self-examination and have lower sensitivity to pain.
- Extraversion, a positive personality trait, is characterized by being a “people person,” directing energies toward other people and the outside world. These individuals exhibit lower pain sensitivity.
(Eisenberg, 2021; Bar-Shalita & Cermak, 2020)
Pain Appraisal and Beliefs
Pain appraisal refers to the meaning ascribed to pain by an individual. Primary appraisal involves evaluation of the significance of the pain as either a threat or irrelevant, and secondary appraisal involves evaluation of the controllability of pain and one’s coping resources. Beliefs refer to assumptions about reality that shape how the person interprets events.
Appraisal and beliefs about the meaning of pain can have a strong impact on an individual’s response to pain. If a pain signal is interpreted as harmful, it may be perceived as more intense or more unpleasant and evoke more escape or avoidance behaviors.
Pain appraisal and pain beliefs are also determinants of adjustment to chronic pain. Pain that is viewed as a signal of damage, leading to disability, uncontrollable, and a permanent condition has been shown to affect an individual’s responses (Ballantyne et al., 2019).
Fear and Catastrophizing
Pain catastrophizing is an exaggerated, negative cognitive and emotional orientation toward actual or anticipated pain experiences. Catastrophizing has been associated with an increased perception of severity and disability in both acute and chronic pain among persons with many different pain diagnoses. Catastrophizing also alters perception of noxious stimulation.
People who experience chronic pain often anticipate that specific activities will increase pain or induce further injury, and these fears may contribute to avoidance of activity and subsequently greater physical deconditioning, emotional distress, and ultimately, greater disability (Ballantyne et al., 2019).
Emotions
Emotion and pain interact in several ways. Emotional distress may predispose a person to experience pain, be a cause of symptoms, be a modulating factor amplifying or inhibiting the severity of pain, be a consequence of persistent pain, or be a perpetuating factor. Emotional distress is commonly observed in people with chronic pain.
Anxiety is common for patients with pain. Up to 45% of patients with chronic pain screen positive for an anxiety disorder. Those with chronic pain who have comorbid anxiety may have a lower pain tolerance, be more prone to medication side effects or fearful of having side effects, and be more fearful of pain itself.
Up to 50% of patients with chronic pain experience depression, and on average, 65% of depressed individuals also report pain symptoms. There is evidence of a strong association between chronic pain and depression, but evidence is lacking as to whether chronic pain causes depression or depression causes chronic pain (Ballantyne et al., 2019).
PAIN ASSESSMENT
A precise and systematic assessment of pain is important for making an accurate diagnosis and for the development of an effective treatment plan. Pain is a multidimensional phenomenon that produces strong emotional reactions that can affect an individual’s function, quality of life, emotional state, social and vocational status, and general well-being. Therefore, it is recommended that pain be assessed using a multidimensional approach and that these various impacts be addressed and included in the diagnostic formulation.
A comprehensive pain assessment includes a history of the pain, behavioral observations, past medical history, medications, family history, a physical examination, and if necessary, diagnostic testing.
Pain History
A pain assessment begins with the history of the problem and can be obtained from written documents and from interviews with the person in pain as well as family members and other caregivers. Pain is a subjective symptom, and pain assessment is, therefore, based on the patient’s own perception of pain and its severity.
ELEMENTS OF PAIN HISTORY
Because pain is subjective, a self-report is considered the “gold standard,” or the best, most accurate measure of a person’s pain. One method to obtain a complete pain history is the PQRST assessment (see box).
PQRST PAIN ASSESSMENT
Provocation/Palliation (P)
- What were you doing when the pain started?
- What caused the pain?
- What seems to trigger it (e.g., stress, position, certain activities)?
- What relieves it (e.g., medications, massage, heat/cold, changing position, being active, resting)?
- What aggravates it (e.g., movement, bending, lying down, walking, standing)?
Quality/Quantity (Q)
- What does the pain feel like (e.g., sharp, dull, stabbing, burning, crushing, throbbing, nauseating, shooting, twisting, stretching)?
Region/Radiation (R)
- Where is the pain located?
- Does the pain radiate, and if so, where?
- Does the pain feel like it travels/moves around?
- Did it start somewhere else and is now localized to another spot?
- Is it accompanied by other signs and symptoms?
Severity Scale (S)
- How severe is the pain on a scale of 0–10, with 0 as no pain and 10 as the worst pain ever?
- Does the pain interfere with activities?
- How bad is the pain at its worst?
- Does it force you to sit down, lie down, slow down?
- How long does an episode last?
Timing (T)
- When or at what time did the pain begin?
- How long did it last?
- How often does it occur (e.g., hourly, daily, weekly, monthly)?
- Is the pain sudden or gradual in onset?
- When do you usually experience it (e.g., daytime, night, early morning)?
- Are you ever awakened by it?
- Does it ever occur before, during, or after meals?
- Does it occur seasonally?
(Crozer Health, 2022)
ASSESSMENT TOOLS
Pain scores have been accepted as the most accurate and reliable measure for assessing patients’ pain and response to pain treatment. Scales have been developed to estimate and/or express the patient’s pain using two methods: unidimensional and multidimensional measures.
Unidimensional pain scales allow the patient to use either words or images to describe their pain. These scales assess a single dimension of pain, typically pain intensity, through patient self-reporting. These are useful for a patient in acute pain when the etiology is known. Examples of unidimensional scales include:
- Numerical rating scale (NRS)
- Visual analogue scale (VAS)
- Verbal rating scale (VRS)
- Faces scale
(UFHealth, 2022)
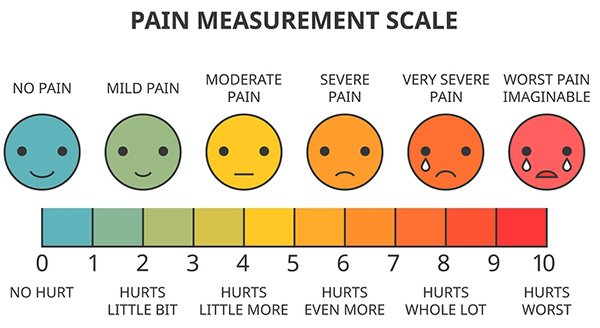
Unidimensional visual analogue scale (VAS) measuring pain intensity. (Source: Lukpedclub/Bigstock.com.)
Multidimensional scales are more complex. They measure pain intensity, its nature or quality, its location, and its impact on mood or activity. These scales are useful in complex or persistent acute or chronic pain. Examples include:
- Multidimensional Pain Inventory
- McGill Pain Questionnaire (MPQ)
- Brief Pain Inventory Short Form (BPI-SF)
(UFHealth, 2022)
Behavioral Observations
Most people who are experiencing pain usually show it either by verbal complaint or nonverbal behaviors or indicators. It is important, however, to remember that people in pain may or may not display behaviors that are considered an indication of “being in pain,” and making judgments about their honesty is inappropriate. The following table lists some typical behavioral and physiologic indicators of pain that healthcare providers may observe when completing a pain assessment.
| Type of Indicator | Examples |
|---|---|
| (Toney-Butler, 2019; Victoria Department of Health, 2021) | |
| Facial expressions |
|
| Vocalizations |
|
| Body movements |
|
| Activity/routine changes |
|
| Social interaction |
|
| Protective movements |
|
| Mental status changes |
|
| Physiologic changes |
|
Medical and Surgical History
Relevant past medical and surgical history may help determine the etiology of pain (e.g., diabetes, history of cancer, rheumatic disease) and may reveal conditions that affect the choice of therapy. This includes:
- Prior medical illness (e.g., renal or hepatic insufficiency/disease, which affects choice of analgesic and dosing)
- Prior psychiatric illnesses (e.g., depression or anxiety)
- Prior surgeries, scarring, repeated surgeries (may increase sensitivity to pain)
- Past injuries and accidents
- Coexisting acute or chronic illnesses
- Chemical dependence
- Prior problems with pain and treatment outcomes
- Investigations conducted (e.g., medical imaging)
A complete list of current medications (past and present) and usage, including over-the-counter medications and alternative, herbal, and natural products, is obtained, as well as the patient’s report of their effectiveness. Evaluation of physiologic tolerance (diminished response) related to chronic use of some medications and use of alcohol and illicit drugs is also included.
Family history is important, as it may give a clue to any predisposition to pain-causing illnesses and conditions that may involve the connective tissues (e.g., kyphoscoliosis), metabolism (e.g., sickle cell disease), and neurologic system (e.g., familial amyloid neuropathy). Other types of disorders that may cluster in families include fibromyalgia, persistent back pain, irritable bowel syndrome, and some types of arthritis (CASN/AFPC, 2021).
Review of Systems
The review of systems may suggest conditions that are associated with nociplastic sensory hypersensitivity (pain with no clear evidence as to source), and may support a syndromic pain diagnosis such as chronic fatigue, headache, or widespread conditions such as fibromyalgia (Tauben & Stacey, 2022).
The psychosocial history is an important aspect to a review of systems, because what first appears to be a simple problem can become much more complex due to the influence of psychological and social factors. A psychosocial history includes:
- Psychological history: emotional state, personality, self-esteem
- History of mental illness and past traumatic experiences
- Family systems
- Social history: economic factors, education, social class, culture/ethnicity
(Caring to the End, 2022)
Functional Assessment
Components of a functional assessment include:
- Ability to complete activities of daily living: Hygiene, dressing, cooking, eating, walking, transferring, toileting, shopping, housework, etc.
- Mood/mental health: Presence of depression, anxiety, social isolation, lack of energy and/or interest in social interactions
- Mobility: Ability to move (with or without assistance), including bed mobility, transfers, ambulation, stair climbing, etc.; use of mobility and/or assistive devices (e.g., walker, cane); and ability to engage in activities enjoyed before experiencing pain
- Work ability: Occupation, usual housework, home maintenance
- Sleep: Patterns, interruptions, medication use
- Relations with other people:
- Serving in a caregiving role (e.g., childcare, eldercare)
- Ability to fulfill family obligations
- Ability to engage in social activities and interactions
- Ability to have intimate relationships
- Support needed and available
Physical Examination
A systematic, targeted, pain-focused physical examination is most fruitful when the pain history interview and behavioral observations are conducted at the same time. Because pain may be referred from some other area of the body, the examination should include a full visual scan from head to toe.
- Mental status examination. This includes cognitive function, mood and affect, thought process and content, judgment, and insight. Signs of mental deterioration should match with the patient’s history or should prompt a search for an underlying pathology.
- Vital signs. They can provide objective information about the patient’s general health status, and, if abnormal, may be a relative contraindication to certain interventions. Vital signs may be elevated when a patient is experiencing acute pain. Elevated temperature may signal an infectious cause for pain.
- General inspection. This begins when the clinician first encounters the patient and notes any obvious sign of pain, such as limping, unusual posture of the body, splinting or guarding, facial expression, vocalizations, and the presence of obesity. The examiner looks at skin color and pigment changes, which may indicate inflammation, sympathetic dysfunction, or a prior herpes zoster eruption. Atrophy may indicate guarding and lack of use or denervation. Poor healing indicates poor perfusion possibly associated with ischemic injuries, diabetic neuropathy, or sympathetic dysfunction. Surgical scars should be identified, particularly in the cervical, thoracic, or lumbar spinal areas.
- Auscultation of the lungs, heart, and bowel sounds. This should be done as part of a routine examination, and especially if pertinent to the complaint.
- Palpation. Touch is used to gather information such as skin temperature, pulses, internal masses, tenderness, or rigidity. The painful area is demarcated, with the clinician feeling for changes in pain intensity within the area, trigger points, and changes in sensory or pain processing. Widespread pain hypersensitivity to palpation may suggest a more complex centralized pain process.
- Musculoskeletal examination. This includes both inspection and palpation for abnormal movements, range of motion, functional limitations, swelling and tenderness of the joints, temperature and color changes, crepitation, and deformity. Inspection of the affected area is done, noting signs of recent trauma as well as evidence of more remote trauma such as scarring. It is important to determine secondary pain, even in patients whose primary source of pain is musculoskeletal. For example, if there is a knee problem, structures that directly affect the function of the knee (such as the low back, hip, foot, ankle, and supporting structures of the knee) are evaluated.
- Neurologic examination. This includes evaluating level of alertness, degree of orientation, behavior and mood, intellectual function, motor system (muscle tone and strength), and balance. A comprehensive sensory examination includes tests for light touch, pinprick, pressure, vibration, joint position, and heat and cold sensation. The examination also involves observation of the individual’s gait, coordination, and balance and testing for abnormal deep tendon reflexes. Hyperreflexia may be indicative of a number of possible conditions and indicate spinal cord myelopathy (e.g., compression, syrinx, or multiple sclerosis).
- Abdominal, pelvic, or rectal examination. This assesses for suspected disease conditions that can cause pain referred to the back, such as pelvic inflammatory disease, endometriosis, or prostatitis.
(Anesthesia Key, 2019; Tauben & Stacey, 2022)
Diagnostic Testing
Although there are no diagnostic tests available as yet to determine how much pain a person is experiencing, and no test that can measure the intensity or location of pain, there are a number of tests that can be done to determine the cause or source of pain.
LABORATORY TESTS
Routine blood studies are not indicated, but directed testing should be ordered when specific causes of pain are suggested by the patient’s history or physical examination.
- Complete blood count (CBC), to detect the presence of an infection and some kinds of cancer
- Comprehensive metabolic panel (CMP), to give a picture of a person’s general health and to consider drug clearance and metabolism in the setting of renal or liver dysfunction that may affect treatment options
- Erythrocyte sedimentation rate (ESR), to assess for inflammation and autoimmune disorders
- C-reactive protein (CRP), to assess for infection, inflammation, and possible elevation due to polymyalgia rheumatica or rheumatoid arthritis
- Vitamin B12, B6, and folate levels, to assess for deficiencies that cause neurologic symptoms
- Fasting blood sugar (FBS) and glycated hemoglobin (HbA1C), to test for diabetes or to monitor control of diabetes
- Hemoglobin S (HbS or Hgb), to test for sickle cell disease
- HIV antibodies (ELISA or Western Blot), to detect HIV infection
- HSV antibodies, to assess for herpes simplex virus infection
- Lyme antibody testing, to rule out Lyme’s disease, which can progress to the joints and peripheral nerves
- Rheumatologic tests (rheumatoid factor, ESR, ANA), to rule out rheumatoid arthritis and other autoimmune diseases (e.g., systemic lupus erythematosus) and infections (e.g., hepatitis, syphilis)
- HLA-B27 antigen, a genetic marker, to rule out ankylosing spondylitis and reactive arthritis
(Nnanna, 2021; Asher, 2022)
IMAGING AND ELECTRODIAGNOSTIC TESTING
- Plain X-ray films, to demonstrate bony pathology and some soft tissue tumors
- Ultrasound, to help diagnose strains, sprains, tears, and other soft tissue conditions
- Myelograms using a contrast injected intrathecally, to assess the spinal cord, subarachnoid space, or other structures for changes or abnormalities
- Computerized tomography (CT), to obtain images that give details of anatomic structures
- Discogram, to view and assess internal structure of a disc to determine if it is the source of pain
- Magnetic resonance imaging (MRI) for superior soft tissue visualization, to diagnose spinal disc disease or neural compression; best for evaluation of spinal alignment and investigation for infections or tumors
- 18-FDG PET and MRI, a newer PET/MRI method, to pinpoint regions responsible for causing pain
- Functional MRI, to provide data on metabolic and functional measurements in addition to anatomic details
- Bone scans, to help diagnose tumors of the bone or metastatic disease, osteomyelitis, fractures, joint disease, avascular necrosis, and Paget’s disease
- Electromyography (EMG), to detect abnormal electrical activity in many diseases and conditions
- Nerve conduction studies (NCS), to measure changes in the peripheral sensory and motor nerves by stimulating them in various places along their courses and to isolate a specific site of injury
- Diagnostic nerve block, which numbs pain in specific nerve locations, thereby allowing the patient’s response to the nerve block, to help determine the cause and site of pain
- Somatosensory evoked potential (SSEP), to assess for generalized disorders of the nervous system (e.g., multiple sclerosis)
- Electroencephalography (EEG) and magnetoencephalography (MEG), to identify neural pathways signaling chronic pain
(Agranoff, 2020; Wheeler, 2021; O’Connor, 2020)
Psychological Examination
A psychological assessment is intended to identify emotional reactions, maladaptive thinking and behavior, and social problems that can contribute to pain and disability. A psychological assessment includes a semistructured clinical interview and self-report instrument to assess differences in the domains of pain experience, functional impairment, and pain-related disability.
There are three main purposes for psychological and psychiatric evaluations related to assessment of pain. These are to:
- Assess the impact of personality, psychiatric, physical, and motivational factors that can affect symptoms of chronic pain
- Measure psychiatric and psychological distress stemming from pain due to an incident or an injury at work
- Determine eligibility for spinal cord stimulator or morphine pump placement
(Comprehensive MedPsych Systems, 2021; Jamison & Craig, 2022)
PAIN AND RISK FOR SUICIDE
Chronic pain is prevalent in people who die by suicide. Chronic, nonmalignant pain, independent of other factors such as sociodemographic and physical and mental health status, doubles the risk of suicide. Risk factors for suicidal ideation and behavior in those with chronic pain include:
- Multiple pain conditions
- Severe pain
- More frequent episodes of intermittent pain (e.g., migraines)
- Longer duration of pain
- Sleep onset insomnia
Psychological processes relevant to patients with chronic pain who may be at risk for suicide include helplessness and hopelessness, a desire to escape the pain, and problem-solving deficit. Evaluation should include patient and family past histories of suicidal ideation and behavior (Schreiber & Culpepper, 2022).
Barriers to Assessing Pain
Optimal comprehensive pain assessment requires removal of barriers in the healthcare system; among healthcare professionals; and in patients, family, and society.
Healthcare professional barriers: Studies have found that nurses rely on physiologic parameters or observed behaviors rather than using formal pain assessment tools. Deficits in knowledge related to pain assessment and management were found most frequently to be related to:
- Behavioral pain indicators
- Perceptions of a patient’s pain tolerance
- Use of verbal and nonverbal pain assessment tools
- Pharmacologic and nonpharmacologic pain management
Although self-reporting is the gold standard for pain assessment, nurses have been found to perceive self-reporting of pain as an inaccurate measure of intensity and tend to encourage patients to endure pain as long as they can before offering analgesia (Rababa et al., 2021).
Another barrier is healthcare provider bias, which may manifest as prejudice against a particular group or the use of stereotypes to categorize a particular patient as not having pain or a particular illness as not causing pain. For instance, research has shown that Black Americans are systematically undertreated for pain relative to White Americans and that this is related to false beliefs such as “Black people’s skin is thicker than White people’s skin” (Hoffman et al., 2016).
Patient and family/caregiver related barriers: The most frequently reported barrier is the patient’s inability to communicate (see also “Assessing Pain in Special Populations” below). Also, the biopsychosocial nature of pain means that a person’s knowledge and personal beliefs about pain and its treatment may greatly influence how well their pain can be managed. A patient history of substance abuse, alcoholism, or suicide attempt may impede pain management as well.
System-related barriers include a lack of standardized assessment forms and tools for critically ill and nonverbal patients, lack of standardized guidelines and protocols for pain evaluation and control, heavy workloads of nurses, and nursing staff shortages (Rababa et al., 2021; Lee, 2022).
Assessing Pain in Special Populations
Accurate pain assessment can be challenging in certain populations, including infants, children, and cognitively impaired individuals, due to communication barriers. Because pain is a subjective experience, being unable to obtain this subjective information can lead to a less-than-optimal assessment.
ASSESSMENT OF PAIN IN NEONATES
Since the 1980s, evidence has shown that preterm and term infants experience pain and stress in response to noxious stimuli. As general rule, anything that causes pain in adults or older children will also cause pain in neonates. Effective neonatal pain assessment is essential for optimal pain management and requires appropriately sensitive and accurate clinical pain assessment tools as well as clinical staff that is trained to detect neonatal pain using such tools.
Neonatal pain assessment tools rely on surrogate measure of physiologic and behavioral response to pain or noxious stimuli. Examples of scales most commonly used for acute pain assessment include:
- PIP-R (Premature Infant Pain Profile-Revised)
- N-PASS (Neonatal Pain Agitation and Sedation Scale)
- NIPS (Neonatal Infant Pain Scale)
- CRIES (crying, requires oxygen, increased vital signs, expression, sleepless)
- NFCS (Neonatal Facial Coding System)
- BIPP (Behavioral Infant Pain Profile)
There are challenges, however, that limit the accuracy of using such tools, as they require evaluation by observers among whom there may be significant variability. These tools also require observation, mental calculation, and recording of 3 to 10 parameters in real time, all while the provider is performing a painful procedure. At this time there is no “gold standard” established for assessment of pain in the neonate (Anand, 2022).
ASSESSMENT OF PAIN IN INFANTS AND CHILDREN
Assessment of pain severity in children is performed by self-report or by behavioral observational scales in those unable to self-report. Self-reporting relies on the cognitive ability to understand that pain severity can be measured along a continuum. Younger children (ages 3 to 8 years) are capable of quantifying pain and translating it to a visual representation. Visual analogue pain scales based on a series of faces showing an increase in distress or pain are used for this age group. The reliability of pain assessment increases with age and cognitive ability of the child.
Assessment of pain in older children (ages 8 to 11 years) is generally performed utilizing visual analogue tools that rate the intensity of pain on a horizontal or numeric scale. Adolescents can also rate pain using a numerical scale, and a description of pain can usually be obtained from pain history.
The following pain-location tools can be used to determine location of pain in both children and adolescents. These tools use a graphic outline of the body, and the patient is asked to color in the area of the body where pain is being experienced:
- Adolescent and Pediatric Pain Tool
- Pediatric Pain Questionnaire
The following observational tools are used for assessing pain in infants and children who are unable to self-report. They are based on facial expressions, ability to be consoled, level of interactions, limb and trunk motor responses, and verbal responses:
- rFLACC (Revised Face, Legs, Activity, Cry, Consolability) for nonverbal children
- NCCPC-PV (Non-Communicating Children Pain Checklist–Postoperative Version) for nonverbal children
- NAPI (Nursing Assessment of Pain Intensity) for newborn to 16 years of age
- PPP (Pediatric Pain Profile) for nonverbal children
- INRS (Individualized Numeric Rating Scale)
Pain can also be assessed by identifying the impact it has on daily life, including participation in school activities, sports, and relationships (Hauer & Jones, 2021).
Extremely immature and chronically ill infants and children who have been exposed to repeated painful experiences have difficulty generating a pain response. Caution should be taken not to interpret this response as an indication that the patient is not in pain. Also, chronic pain can sap energy, causing an infant or child to be withdrawn and become still and quiet (Martin et al., 2019).
In children ages 3 to 4 years, self-report measures may be used. However, children may underreport their pain to avoid future injections or other procedures aimed at alleviating pain (Kishner, 2018).
ASSESSMENT OF PAIN IN THE COGNITIVELY IMPAIRED
Many conditions can lead to cognitive impairment that can make pain assessment difficult, such as head trauma, memory deficits, unconsciousness, and delirium. Dementias are the leading cause of impaired cognition in older adults. These individuals may have communication barriers and challenges when complex pain assessment tools are used. In these instances, behavioral observation–based assessments are optimal. Behaviors include:
- Facial expressions (e.g., frowning, grimacing, rapid blinking)
- Verbalizations/vocalizations (e.g., moaning, sighing, verbal abuse)
- Body movements (e.g., rigid, tense, guarding, fidgeting, inactive, pacing)
- Altered interpersonal interactions (e.g., aggression, resistance to care, disruption, withdrawal)
- Activity patterns (e.g., changes in appetite or sleep, cessation of regular routines)
- Mental status changes (e.g., increased confusion, irritability)
There are various pain rating scales available, with none yet shown to be clearly superior. Clinicians, therefore, should choose one tool and use it consistently to ensure uniformity among healthcare providers across shifts. Examples include:
- Doloplus-2
- Assessment of Discomfort in Dementia Protocol (ADDP)
- Pain Assessment in Advanced Dementia (PAINAD)
- Checklist of Nonverbal Pain Indicators
- Pain Assessment for the Demented Elderly
- Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC)
- Abbey pain scale
(Wilner & Arnold, 2022)
ASSESSMENT OF PAIN IN THE OLDER ADULT
Pain is prevalent in older persons, and this increases with age, but pain in the absence of disease is not a normal part of aging. Pain is of significant intensity in about one fifth of older adults, and pain is the most common reason for an older person to consult a physician.
Issues that can make assessment of pain in this population difficult include comorbidities, polypharmacy, and cognitive dysfunction. Older adults may believe that their pain is a normal part of aging or that it cannot be treated, or they may not report it. They may also be concerned that treatment will lead to expensive tests and/or increased medications.
Multiple accompanying medical comorbidities can make it difficult to distinguish acute pain caused by a new illness from that of an existing condition. It is important to learn what the patient’s baseline level of functioning is, and obtaining a focused history will help determine this.
Communication may be impaired as a result of decreased hearing and vision, which may limit verbal communication as well as the use of written pain assessment tools. Recognizing that some patients require extra time to consider a posed question and formulate an answer and speaking more slowly or distinctly are important considerations.
Family members, advocates, or caregivers can provide information about the patient’s baseline cognitive and physical functioning and can validate history. They may also provide some of the best evidence for heightened or chronic pain, which can include increased agitation; changes in functional status, body posture, or gait; and social isolation.
The best pain assessment is obtained by using a standardized tool validated for use in the older adult. It should be sensitive to cognitive, language, and sensory impairments. Commonly used assessment tools for this age group include:
- Visual analogue scales
- Numeric rating scales
- McGill Pain Questionnaire
- Pain Attitudes and Beliefs Scale
- Brief Pain Inventory
- Geriatric Pain Measure
(Buowari, 2021)
STRATEGIES FOR TREATING AND MANAGING PAIN
A comprehensive pain management approach includes:
- Appropriate pharmacologic and nonpharmacologic interventions
- Education of patient, family, and caregivers about the plan
- Ongoing assessment of treatment outcomes
- Regular review of the treatment plan
Pharmacologic Interventions
Pharmacologic interventions can be broadly categorized as primary analgesic medications and adjuvant (co-analgesic, or “helper”) medications. Analgesics include nonopioid analgesics and opioid analgesics. Nonopioids are non-narcotic analgesics used to treat mild pain and also to serve as adjuvant medication for relief of moderate to severe pain. Opioids are narcotics used for moderate to severe pain. Cannabinoids are a unique class of drugs that may be used for pain and do not fit into these categories.
NONOPIOID ANALGESICS
Nonopioid analgesics include acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs). Many are available over the counter; some are available by prescription only.
Acetaminophen
Acetaminophen is a pain reliever and a fever-reducing agent widely used to treat both acute and chronic pain. Acetaminophen is a p-aminophenol derivative whose exact mechanism is not yet fully known. It may inhibit the nitric oxide pathway mediated by a variety of neurotransmitter receptors, resulting in elevation of the pain threshold. The antipyretic activity may result from inhibition of prostaglandin synthesis and release in the central nervous system and prostaglandin-mediated effects on the heat-regulating center in the anterior hypothalamus.
Acetaminophen is harmless at low doses but has direct hepatotoxic potential when taken as an inadvertent overdose (e.g., patients not recognizing the presence of the drug in multiple over-the-counter and/or prescription products being taken), and can cause acute liver injury and death from acute liver failure. Even in therapeutic doses, acetaminophen can cause transient serum aminotransferase elevations.
In the United States, acetaminophen is sold under the brand name Tylenol and is used to provide temporary analgesia in the treatment of mild to moderate pain. Acetaminophen is also used in fixed combination with other agents for short-term relief of minor aches and pain.
Injectable acetaminophen (Ofirmev) is indicated for:
- Management of mild to moderate pain in adult and pediatric patients ages 2 years and older
- Management of mild to moderate pain with adjunctive opioid analgesics in adults and pediatric patients ages 2 years and older
- Reduction of fever in adult and pediatric patients
(NLM, 2022a)
Nonsteroidal Anti-Inflammatory Drugs
There are more than 20 different NSAIDS available over the counter or by prescription. There are two main types of NSAIDS: nonselective and selective. Nonselective NSAIDS commonly available without prescription include aspirin, ibuprofen (Advil, Motrin), and naproxen (Aleve). Selective NSAIDs, also called COX-2 inhibitors, are as effective in relieving pain and inflammation as nonselective NSAIDs but are less apt to cause gastrointestinal injury. Celecoxib (Celebrex) is the only COX-2 inhibitor available in the United States.
NSAIDs can be administered orally, rectally, parenterally, and topically. Side effects can include:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Decreased appetite
- Rash
- Dizziness
- Tinnitus (ringing in the ears) in those who take high doses of aspirin
- Headache
- Drowsiness
- Kidney failure (primarily with chronic use)
- Liver failure
- Ulcers
- Prolonged bleeding after injury or surgery
(Solomon, 2022)
NSAIDs (with the exception of low-dose aspirin) may increase the risk of elevated blood pressure, potentially fatal heart attacks, and stroke (Curfman, 2019).
| Classification | Generic name (Brand name) |
|---|---|
| Salicylates |
|
| Acetic acids |
|
| Proprionic acids |
|
| Fenamates |
|
| Oxicam derivatives |
|
| COX-2 inhibitor |
|
OPIOID ANALGESICS
Opioid analgesics are human-made drugs that are chemically similar to opiates found in the seedpod of the poppy (Papaver somniferum).
Opiates are refined from the natural plant matter and include:
- Opium
- Morphine
- Codeine
- Heroin
Opioids are synthesized compounds using thebaine, an alkaloid extracted from Papaver bracteatum (Persian poppy). Examples include:
- Oxycodone
- Hydrocodone
- Oxymorphone
- Naloxone
- Buprenorphine
Drugs that are created in laboratories that mimic effects of opiates but are not derived from the opium poppy are synthetized drugs. Examples include methadone, fentanyl, and meperidine (OR ADPC, 2022).
Opioid Receptors and Mechanism of Action
Opioid receptors are found in the central nervous system, pituitary gland, gastrointestinal tract, grey matter of the brain, and dorsal horn of the spinal cord. Opioid analgesics produce pain relief by acting on these central and peripheral opioid receptors to inhibit the transmission of nociceptive input and the perception of pain. There are four types of opioid receptors, which produce the following effects:
| Type | Effects |
|---|---|
| (Dhaliwal & Gupta, 2021) | |
| Mu | Mu-1
|
| Delta |
|
| Kappa |
|
| Nociceptin |
|
Opioid Classifications
Opioids are classified by the effect (intrinsic activity) they have on the mu receptors and include full agonists, partial agonists, and antagonists.
Full agonists are opioid drugs that bind to mu opioid receptors and cause them to produce endorphins, which provide pain relief, and depending on the dose and frequency, addictive effects and feelings of euphoria. Examples of full agonists are oxycodone, methadone, codeine, heroin, and morphine.
Partial agonists are drugs that bind primarily to mu opioid receptors and cause them to produce endorphins but to a much lesser extent than full agonists. When the dosage of a partial agonist is increased, there is only a small increase, if any, in the production of endorphins. Buprenorphine/naloxone (Suboxone) and buprenorphine (Subutex) are partial agonists.
Antagonists are drugs that bind to the mu opioid receptors but have no intrinsic activity and prevent other opioids from stimulating the mu receptors and producing endorphins. Naloxone and naltrexone are opioid antagonists (WSHCA, 2021).
| Generic | Brand Name(s) |
|---|---|
| (U.S. FDA, 2022) | |
| Fentanyl |
|
| Hydrocodone |
|
| Hydrocodone/acetaminophen | n/a |
| Hydrocodone/ibuprofen |
|
| Hydromorphone |
|
| Meperidine |
|
| Methadone |
|
| Morphine |
|
| Oxycodone |
|
| Oxycodone/acetaminophen |
|
| Oxycodone/aspirin |
|
| Benzhydrocodone/acetaminophen |
|
Adverse Effects of Opioid Analgesics
Both short- and long-term use of opioids is associated with a high rate of adverse effects involving multiple body systems. Such adverse effects can occur at all dose ranges (see table).
| Body System | Effects | |
|---|---|---|
| (Portenoy et al., 2022a; Mandall, 2019) | ||
| Central nervous system |
|
|
| Neuroendocrine |
|
|
| Respiratory |
|
|
| Cardiovascular |
|
|
| Gastrointestinal |
|
|
| Genitourinary |
|
|
| Biliary |
| |
| Skin and eye |
|
|
| Immune system |
|
|
| Other |
|
|
Opioids and Managing Breakthrough Pain
Most patients with chronic pain due to advanced disease report having episodic pain referred to as breakthrough pain. Breakthrough pain is a transitory, severe, acute pain that occurs in patients with chronic pain that has been adequately controlled by an opioid regimen.
Breakthrough pain includes the following:
- Incident pain occurs with specific activities and can be predicted. Pain management requires a proactive approach using a quick-acting, short-term-lasting pain medication before the patient is involved in those activities. Dosage is adjusted based on the level and duration of the activity that is expected to cause pain.
- Spontaneous pain is unpredictable, not associated with any specific activity, and more difficult to treat. A quick-acting, short-term-lasting pain medication is given as soon as the patient feels pain. Better control of pain may result from use of adjuvant medications.
- End-of-dose medication failure is pain that occurs toward the end of the timeframe in which the medication is intended to be effective. The treatment may involve shortening the interval between scheduled doses or increasing the dose.
Breakthrough pain episodes are typically managed with a short-acting oral opioid drug, referred to as a rescue dose, taken on an as-needed basis in conjunction with the fixed-schedule, long-acting medication. A typical dose for rescue is 5%–15% of the basal daily requirement of opioid.
Breakthrough pain may also be treated with one of the newer rapid-onset, transmucosal fentanyl formations. There are several formulations available in the United States:
- Actiq (oral transmucosal fentanyl lozenge)
- Abstral (immediate-release transmucosal tablet)
- Fentora (effervescent fentanyl buccal tablet)
- Lazanda (nasal spray)
- Subsys (sublingual spray)
To prescribe any of these drugs, clinicians must complete online education. Each patient treated requires registrations of the patient, the prescribing clinician, and the pharmacist. Additional regulations now require that opioid tolerance be verified and documented by both the prescriber and the outpatient pharmacy prior to each individual prescription. Because of the cost and limited experience, the transmucosal drugs are generally considered only after a patient has demonstrated a poor response to an oral rescue dose (Portenoy et al., 2022b).
Opioids and Drug Tolerance, Dependence, and Addiction
When an opioid drug is used on a regular basis, generally after more than 2–3 weeks, the same dose of the drug has less of an effect. This is referred to as tolerance. A person who is developing tolerance may require larger amounts of the drug to get the same effect. Tolerance levels vary between individuals and occur when parts of the body affected by the drug begin to respond less to repeated stimulation and the number of cell receptors the drug attaches to decrease.
Opioid use also affects the brain’s production of dopamine, which creates a euphoric high, causing the release of large amounts of the neurotransmitter. Over time, the brain will rely on the drug for dopamine production. With repeated use of opioids and the development of tolerance, dependence occurs. Dependence is characterized by the symptoms of tolerance and withdrawal. The brain adapts to repeated exposure to the drug and can only function normally in the presence of the drug. When the drug is withdrawn, physiologic reactions occur, which can be mild or even life-threatening. Withdrawal symptoms are described in the table below.
| Type | Symptom |
|---|---|
| (Sevarino, 2022) | |
| Gastrointestinal |
|
| Flu-like symptoms |
|
| Sympathetic nerve and central nervous system arousal |
|
| Other |
|
Addiction is a disorder with biological, psychological, social, and environmental factors that influence its development and maintenance. About half the risk for addiction is genetic. Genes affect the degree of reward individuals experience when using a drug, as well as how the body processes the substance. Brain changes include alterations in the prefrontal cortex and limbic system involving the neurocircuitry of reward, motivation, memory, impulse control, and judgement. This may lead to increased cravings for a drug as well as impairment in the ability to regulate this impulse (Bukstein, 2022; APA, 2022; Sevarino, 2022).
Opioid Overdose
Due to their pharmacologic effects, opioids in high doses can cause respiratory depression and death. Most drug-related deaths worldwide are attributable to opioids. An opioid overdose can be identified by a combination of three signs and symptoms, referred to as the opioid overdose triad, which include:
- Pinpoint pupils
- Unconsciousness
- Respiratory depression
Combining opioids with alcohol and sedative medication increases the risk of respiratory depression; and combinations of opioids, alcohol, and sedatives are often present in fatal drug overdoses (WHO, 2022a).
ADJUVANT ANALGESICS
Adjuvant analgesics (co-analgesics) are drugs that were developed for clinical uses other than pain but are used as an analgesic in select circumstances. The following table describes common adjuvant analgesics.
| Class / Indications / Primary Effects | Drugs |
|---|---|
| (Portenoy et al., 2022) | |
| Antidepressants: Neuropathic pain, burning sensation; improves sleep, enhances mood and analgesic effects |
|
| Anticonvulsants: Neuralgic and neuropathic pain; sharp, prickling, shooting pain |
|
| Antispasmodic: Reflex sympathetic dystrophy syndrome (a disorder of the sympathetic nervous system causing chronic, severe pain) |
|
| Antihypertensives: Fibromyalgia, spasticity |
|
| Osteoclast inhibitors: Bone pain |
|
| Radiopharmaceuticals: Bone pain |
|
| Anxiolytics: Help manage anxiety and pain by encouraging muscles to relax |
|
| Neurotoxin: Migraine headache, other focal pain syndromes |
|
| Topical anesthetics: Neuralgic, neuropathic, and musculoskeletal pain |
|
| Corticosteroids: Inflammatory conditions, metastatic bone pain, neuropathic pain, and visceral pain |
|
| Anesthetic drugs: Neuropathic pain, phantom leg pain |
|
| Cannabinoids: Neuropathic pain |
|
| Anticholinergics: Bowel obstruction |
|
ROUTES OF ANALGESIC ADMINISTRATION
Analgesics can be administered by many routes. Each has advantages and disadvantages as well as indications and contraindications. The overriding considerations are effectiveness and safety. The table below lists some of the most common routes for the administration of analgesic drugs.
| Route | Advantages | Disadvantages |
|---|---|---|
| (Doctors.net/uk, n.d.; KnowledgeDose, 2020; Kim & DeJesus, 2022) | ||
| Oral (PO, or per os) |
|
|
| Rectal (R) |
|
|
| Sublingual (SL) and buccal |
|
|
| Intramuscular (IM) |
|
|
| Intravenous (IV) bolus |
|
|
| Continuous intravenous (IV) infusion |
|
|
| Patient-controlled analgesia (PCA) |
|
|
| Subcutaneous (SC) opioid infusion |
|
|
| Intraspinal (neuraxial), intrathecal, epidural, subarachnoid, intraventricular |
|
|
| Regional nerve blocks |
|
|
| Topical (cream-laden anesthetic) |
|
|
| Transdermal skin patch |
|
|
| Nasal sprays |
|
|
CANNABIS (MEDICAL MARIJUANA)
More than two thirds of the states, including Oregon, and the District of Columbia have legalized cannabis for medical treatments. However, federal law continues to prohibit use of cannabis or its derivatives for any purpose. This means that people may be arrested and charged with possession even in states where marijuana use is legal (UGA, 2020; NIDA, 2020a; ASA, 2021).
Under the Controlled Substances Act (CSA), cannabis is classified as a Schedule 1 drug. This means that the federal government views cannabis as highly addictive and that it has no medical value. Doctors may not prescribe cannabis for medical use under federal law, although they can “recommend” its use under the First Amendment (Stonebraker, 2022).
Mechanism of Action
Marijuana is a greenish-gray mixture of the dried flowers of the plant Cannabis sativa. The main psychoactive chemical in marijuana responsible for the intoxicating effects people experience is delta-9-tetrahydrocannabinol (THC). THC’s chemical structure is similar to anandamide, an endogenous cannabinoid, and because of this similarity, it is able to attach to and activate cannabinoid receptors in brain areas that influence pleasure, memory, thinking, and concentration (hippocampus and orbitofrontal cortex); and balance, posture, coordination, and reaction time (cerebellum and basal ganglia). THC, acting through the cannabinoid receptors, also activates the brain’s reward system to release dopamine at levels higher in response to pleasurable behaviors than those in response to other stimuli.
The other chemical from the marijuana plant that is of medical importance is cannabidiol (CBD), thought to be useful in reducing pain and in controlling epileptic seizures. The therapeutic effects of CBD are as an anticonvulsant, antipsychotic, anxiolytic, neuroprotective, sleep-promoting, and anti-inflammatory agent.
CBD, however, does not cause intoxication or euphoria (the “high”) that comes from THC because it does not affect the same receptors as THC. CBD influences the body to use its own endocannabinoids more effectively by inhibiting absorption by the body of the endogenous cannabinoid anandamide, which is associated with regulating pain. Increased levels of anandamide in the blood may reduce the amount of pain a person experiences. CBD also reduces anxiety by changing the shape of the GABA-A receptor in a way that amplifies the natural calming effect of GABA (NIDA, 2020a; Mlost et al., 202; Project CBD, 2022).
CBD is also used for the treatment of epilepsy. The exact mechanism in which CBD creates anticonvulsant effects is not known, but it possesses affinity for multiple targets resulting in functional modulation of neuronal excitability relevant to diseases such as epilepsy (Gray & Whalley, 2020).
FDA-Approved Medical Marijuana Analgesic Formulations
Whether or not the use of marijuana has therapeutic benefits that outweigh its health risks is still unresolved, but marijuana-based medications have been approved or are undergoing clinical trials. Currently the FDA has approved dronabinol (Mariol, Syndros) and nabilone (Cesamet) for relief of chronic nerve-related pain. Nabiximols (Sativest), a mouth spray for treating spasticity and neuropathic pain, is undergoing trials (UGA, 2020; ASA, 2021).
MEDICAL MARIJUANA IN OREGON
Oregon law allows attending providers to recommend the use of medical marijuana for the following medical conditions:
- Cancer
- Glaucoma
- A degenerative or pervasive neurological condition
- HIV/AIDS
- Posttraumatic stress disorder (PTSD)
-
A medical condition or treatment for a medical condition that produces one or more of the following:
- Cachexia (a weight-loss disease that can be caused by HIV or cancer)
- Severe pain
- Severe nausea
- Seizures, including but not limited to seizures caused by epilepsy
- Severe pain
- Persistent muscle spasm, including but not limited to spasms caused by multiple sclerosis
Patients can possess 24 ounces of usable cannabis and can raise 6 mature and 18 immature seedlings. There are also state-licensed dispensaries available (NORML, 2022).
Nonpharmacologic Interventions
Evidence-based nonpharmacologic therapies are safe when correctly administered and can be effective components of comprehensive pain management that can reduce the need for opioids. Nonpharmacologic therapies can be the sole intervention, or they can be combined with other treatments. Nonpharmacologic interventions include physical, psychological, and mind-body modalities.
PHYSICAL MODALITIES
Physical modalities for relief of pain refer to any therapeutic medium that uses the transmission to or through the patient of thermal, electrical, acoustic, radiant, or mechanical energy.
Thermal Modalities (Heat and Cold)
Cold (cryotherapy) is often the first treatment applied to new injuries. Cold causes vasoconstriction, which slows blood flow and leakage of fluid from capillaries into surrounding tissue spaces and reduces bruising, swelling, inflammation, and muscle spasms. Cold slows down the pain messages transmitted to the brain and numbs tissue, acting as a local anesthetic.
Cold therapy can be applied via cold compresses, chemical cold packs, ice packs, immersion or soaking in cold water, massaging the area with an ice cube or ice pack, cold air and vapocoolant sprays, and manual and electric cold compression units. More recently, whole-body cryotherapy has been used for persistent pain in patients with rheumatological conditions; more research is needed to understand the effect on the body and its relation to pain (Physiopedia, 2022b).
Thermal modalities include superficial and deep heat. Heat may facilitate tissue healing, relax skeletal muscles, and decrease spasms and pain. Superficial heat (thermotherapy) is the use of an agent that causes temperature increase and subsequent physiological changes to the superficial layers of the skin, fat, tissues, blood vessels, muscles, nerves, tendons, ligaments, and joints. Superficial heat penetration is usually less than 1 cm into the skin and subcutaneous tissue.
Heat therapy promotes pain relief, vasodilation, increased metabolism, and elasticity of connective tissues, and is used for subacute to chronic conditions. Commonly used superficial heat modalities include hot packs, heat wraps, heating pads, hydrotherapy, steam baths, saunas, paraffin bath, infrared, ultrasound, and fluid therapy (Seidel et al., 2021).
Deep heat is produced when energy is converted into heat as it passes through body tissues. Deep heat can penetrate 3 cm to 5 cm or more without overheating underlying subcutaneous tissue or skin (Hoenig & Cary, 2021). (See also “Acoustic Modalities” below.)
Manual Modalities
Massage is the use of touch or force to areas and tissues for therapeutic purposes. Therapeutic massage involves the application of hands or elbows with the intention of solving a physical problem. Research supports the benefits of massage therapy for pain management, decreasing anxiety and depression, and reducing pain intensity in patients undergoing surgical procedures. Massage reduces pain by stimulating A-beta fibers, resulting in closing of the “gate” to impulses from the periphery, and also stimulates the release of endorphins.
Types of massage include:
- Acupressure: Application of pressure to acupuncture points
- Deep-tissue: Massage to reduce pain and inflammation
- Rolfing and myofascial release: More aggressive techniques that direct force into dysfunctional muscle and fascial tissue
- Neuromuscular therapy: A technique that releases trigger points within tight muscles
- Reflexology: Based on a system of points on the hands, feet, and ears that correspond to other parts of the body; similar in theory to acupressure, applying pressure to these points to stimulate the flow of energy, thus helping to release pain or blockages throughout the body
- Reiki: Use of light touch designed to work with the body’s energy
- Whirlpool: Water massage to decrease muscle tension, improve circulation, and relieve pain
- Swedish: The most common type of massage, to decrease muscle tension, pain, stress, and depression
(Madore, 2022; AMTA, 2022)
Manipulation therapy is an evidence-based practice involving the application of pressure to the spine or other parts of the body to adjust and correct alignment for the treatment of musculoskeletal pain. Such techniques are commonly used to improve pain and function by osteopathic physicians, chiropractors, and physical therapists. Spinal manipulation, for example, is often recommended for acute, subacute, and chronic low back pain as well as osteoarthritis. It has also been found beneficial for neck pain, cervicogenic headache, sports injuries, and prophylaxis of migraine (PPM, 2019).
Acupuncture
Acupuncture involves placing thin needles into targeted areas of the body to ease chronic pain. According to traditional Chinese medicine, the body has patterns of energy (chi) flow. Fine needles are positioned at specific locations on the body to correct or maintain this flow. Modern medicine emphasizes how acupuncture needles stimulate nerve and muscle cells, reducing the sensation of pain and releasing the body’s endorphins. In support of this theory, there is evidence that opioid antagonists block the analgesic effects of acupuncture. Acupuncture needles can be moved or turned once they are in place, and mild electrical pulses are sometimes used between two needles (electro-acupuncture) to expand the area of pain relief (Mayo Clinic, 2022; Ahn, 2020).
Electro-Physical Agents
Electrotherapy is used to treat a range of chronic pain conditions by directing mild electric current to underlying structures.
Transcutaneous electrical nerve stimulation (TENS) is a commonly used device designed specifically for pain relief. TENS provides a low-voltage electrical current through the skin to sensory nerve fibers, producing numbness or tingling sensations that “mask” or “override” sensations of pain. The impulses from TENS fill the nerve pathways and prevent the transmission of pain signals to the brain. It may also stimulate nerves to produce endorphins, which may block the perception of pain. TENS is most commonly used to treat conditions involving muscle, joint, or bone, such as osteoarthritis, fibromyalgia, bursitis, and back and neck pain.
Although TENS may help relieve pain for some people, its effectiveness has not been proven. Many studies have been done on TENS, but most have been small or not well designed. For this reason, some experts claim that TENS can give short-term pain relief but that long-term relief has not been proven (URMC, 2022).
Interferential stimulation is more complex than TENS. It uses dual-frequency stimulation to create circuits that cross over each other to produce maximum pain signal interference at the treatment target site.
Percutaneous electric nerve stimulation (PENS) therapy uses thin needle electrodes that pierce the skin and get closer to nerve endings or muscle than does TENS therapy. PENS therapy does not destroy the affected nerves but makes them less sensitive to pain signals. It is often used if TENS therapy is unsuccessful and to treat diabetic peripheral neuropathy.
Pulse electromagnetic field stimulation (PEMF) therapy uses short bursts of low-level electromagnetic radiation to stimulate nerve or muscle. This radiation works with the body’s natural magnetic field to help increase electrolytes and ions, which naturally influences electrical changes on a cellular level and influences cellular metabolism. This combines with the body’s own recovery processes to help relieve chronic pain (Williams, 2021).
Iontophoresis allows medication to be delivered into and through the skin to a painful area without having to be injected or taken orally. Liquid medication is placed on a patch that is then applied to the painful area. A device, similar to a battery, is then attached to the patch, and the medication is delivered by a mild electrical current. Iontophoresis has been used successfully to anesthetize an area of skin with lidocaine and to treat bursitis, plantar fasciitis, or tendonitis with anti-inflammatory drugs. Although evidence is limited, studies to date indicate this modality may generally be no more effective than placebo (NLM, 2022b; Hoenig & Cary, 2021).
Acoustic Modalities
Therapeutic ultrasound is a form of mechanical energy. The ultrasound device converts electrical energy to high-frequency sound waves that penetrate deeply into muscle, nerve, bone, and connective tissues. The waves vibrate cell molecules and cause friction, which creates heat. Ultrasound can be focused on tissues deep within the body without affecting other tissues close to the surface. It can be used to treat a wide range of health problems but is most commonly used for problems in muscle tissue. The heating effect of ultrasound helps heal muscle pain and reduce chronic inflammation. Currently, there is ongoing debate as to the effectiveness of this modality (Brennan, 2021; Murphy, 2020).
Shortwave diathermy (SWD) produces deep heating by converting high-frequency, alternating electromagnetic energy to thermal energy (friction). Diathermy heats more deeply than hot packs and can heat a larger area than ultrasound. SWD can be either pulsed or continuous. Pulsed shortwave diathermy is used for patients with some acute and subacute conditions, and it prevents tissue temperature from increasing too quickly or too high. Continuous shortwave diathermy increases subcutaneous tissue temperature, and its use is generally limited to chronic conditions. It is usually applied for 20 minutes at the maximum tolerable dose and is used most commonly for short-term musculoskeletal pain relief. SWD penetrates bone and does not pose a risk of periosteal burning.
Microwave diathermy (MWD) does not penetrate as deeply as shortwave diathermy and can be focused more easily than can shortwave diathermy. Both SWD and MWD can create hotspots. MWD is useful in the treatment of traumatic and rheumatic conditions affecting superficial muscles, ligaments, and small superficial joints (Seidel et al., 2021).
Phonophoresis uses ultrasound’s high-frequency sound wave to drive a medication into the tissues by increasing cell permeability for deep heat. A topical anti-inflammatory agent is blended into the ultrasound gel and applied to the skin over the treatment area, and the ultrasound waves carry it into the tissues to reduce inflammation and also provide the benefits of therapeutic heat and vibration (Hoenig & Cary, 2021).
Vibroacoustic therapy (VAT) involves using musical sound waves to aid and facilitate in the relaxation response. VAT causes tissue to resonate, which results in physical changes such as increased blood circulation and metabolism and reduction in sympathetic activity. It is commonly used for anxiety/stress, muscular tension, fatigue, pain management, and other conditions such as fibromyalgia, tinnitus, and Parkinson’s disease (Seidel et al., 2021).
Light Therapy
Light therapy includes low-level laser therapy (LLLT), also known as cold laser therapy, and ultraviolet light (UV). UV light therapy uses the electromagnetic wavelength between X-ray and visible light to bring about a biochemical response in tissue to reduce pain. LLLT uses low-powered laser light to produce this effect.
Photons in light temporarily create a neural blockage (as an anesthetic) by decreasing mitochondrial membrane potential and ATP, which decreases the inflammatory neuropeptides. LLLT is believed to affect fibroblast function, accelerate connective tissue repair, and may have an anti-inflammatory effect by reducing prostaglandin synthesis. UV light may have similar biological effects. Both forms of light therapy are used to decrease acute and chronic pain and inflammation, stimulate collagen metabolism, and promote wound healing (Seidel et al., 2021).
Interventional Pain Modalities
When noninvasive strategies are insufficient, patients may be offered various invasive options that include:
- Injection therapies
- Soft tissue and joint injections may be used for conditions such as bursitis, tendonitis, arthritis, osteoarthritis, and carpal tunnel syndrome. Trigger point injections are used for musculoskeletal pain. Anti-inflammatory medications (corticosteroids) are the most common drugs to use in injections and often are combined with pain relievers such as lidocaine.
- Nerve blocks provide temporary pain relief by injecting a local anesthetic to temporarily interrupt peripheral nerve transmission of pain. Nerve blocks may be given in the facet joints of the spine, hip joint, sacroiliac joint, coccyx, shoulder, elbow, hand, knee, ankle, foot, and occipital, saphenous, and pudendal nerves.
- Neurolytic blocks produce analgesia by destroying afferent neural pathways or sympathetic structures involved in pain transmission by injecting a material that damages the nerve (e.g., water, hypertonic saline, phenol, or alcohol).
- Epidural steroid injections send steroids directly to an inflamed nerve root; two or three injections are required for maximum relief.
- Botulinum toxin injections block chemical signals from nerves that cause muscle to contract. They are used for cervical dystonia and treatment of chronic migraine.
- Prolotherapy is used to treat joint and muscle pain. It is sometimes called regenerative injection or proliferation therapy. It involves injecting a sugar or saline substance into a joint or muscle, where it acts as an irritant, resulting in immune cells and other chemicals being sent to the area, thus starting the body’s natural healing process.
- Platelet-rich plasma (PRP) injections are used for a range of conditions, including musculoskeletal pain and injuries. Platelets contain growth factors that can trigger cell reproduction and stimulate tissue regeneration. An increased concentration of growth factors has been shown to stimulate or speed up the healing process and decrease pain.
- Radiofrequency ablation involves using X-ray guidance to insert a needle with an electrode at the tip, which is then heated in order to temporarily “turn off” a nerve’s ability to transmit pain signals to the brain. Other names are radiofrequency rhizotomy and neuroablation.
- Cryotherapy ablation uses medical-grade nitrous oxide to generate extremely cold temperatures to selectively destroy nerve tissue and prevent transmission of the pain signal to the brain.
- Intrathecal pump implants provide potent medications directly to the source of pain. This is a type of neuromodulation that interrupts pain signals to the brain. It involves a small pump implanted under the skin that is programmed to deliver a specific amount of medication. The pump requires refilling every few months.
- Spinal cord stimulator. A device is implanted under the skin and sends a mild electric current to the spinal cord. Thin wires carry current from a pulse generator to the nerve fibers of the spinal cord. When turned on, the device stimulates nerves in the area where the pain is felt. Pain is reduced because the electrical pulses modify and mask the pain signal.
- Intradiscal electrothermal therapy (IDET) is a minimally invasive technique for treating discogenic low back pain. It involves the percutaneous threading of a flexible catheter into a disc under fluoroscopic guidance. The catheter heats the posterior annulus of the disc, causing contraction of collagen fibers and destruction of afferent nociceptors.
- Dry needling is a physical therapy technique (where allowed by state law) that is part of a larger treatment plan for musculoskeletal pain. Dry needling involves penetrating the skin with a thin filiform needle and stimulating underlying myofascial trigger points and muscular connective tissues that cannot be manually palpable. The goal is to release or inactivate trigger points to relieve pain or improve range of motion.
(Mayo Clinic, 2021; UCSF, 2022; Brennan, 2021; Johns Hopkins Medicine, 2022;Thiyagarajah, 2020; APTA, 2021)
PSYCHOLOGICAL MODALITIES
Cognitive-Behavioral Therapy (CBT)
One of the most common types of psychotherapy used in pain management is cognitive-behavioral therapy. CBT can be described as the “gold standard” psychological treatment for persons with a wide range of pain issues. It can be used alone or in conjunction with medical or interdisciplinary rehabilitation treatments. Currently, CBT is the prevailing psychological treatment for individuals with chronic pain issues.
CBT practice varies but often includes relaxation training, setting and working toward behavioral goals, behavioral activation, guidance in activity pacing, problem-solving training, and cognitive restructuring. The role of CBT is to help patients recognize the emotional and psychological factors that influence pain perception and the behaviors associated with having pain (Physiopedia, 2022c).
Acceptance and Commitment Therapy (ACT)
The basic premise of acceptance and commitment therapy is for patients to shift their primary focus from reducing or eliminating pain to fully engaging in their lives. The goal of the therapy is to help patients accept whatever discomfort exists, both physical and emotional, while continuing to live their lives according to their values. ACT applies six core treatment processes to create psychological flexibility (Glasofer, 2021).
MIND-BODY TECHNIQUES
Biofeedback
Biofeedback is the use of instrumentation to mirror psychophysiologic processes, such as blood pressure, heart rate, and skin temperature, of which an individual normally is unaware and which may be brought under voluntary control. Researchers aren’t exactly sure how or why biofeedback works, but they do know that it promotes relaxation, which can help relieve many conditions related to stress (Watson, 2020).
Relaxation Therapies
Relaxation therapies have been found helpful in the management of chronic headaches and other types of chronic pain. Relaxation encourages reduction in muscle tension, resulting in a decrease in pain intensity. There are a number of practices—such as progressive relaxation, autogenic training, guided imagery, self-hypnosis, and deep-breathing exercises—and the goal is similar for all: to produce the body’s natural relaxation response, characterized by slower breathing, lower blood pressure, and a feeling of increased well-being (NCCIH, 2021).
Hypnosis
Hypnosis is a procedure involving cognitive processes in which the patient is guided by a health professional to respond to suggestions for changes in perceptions, sensations, thoughts, feelings, and behaviors. It involves learning how to use the mind and thoughts to manage emotional distress, unpleasant physical symptoms, and certain habits or behaviors. Hypnosis can provide analgesia, reduce stress, relieve anxiety, improve sleep, improve mood, and reduce the need for opioids. It can also enhance the effectiveness of other forms of relaxation therapies and biofeedback for pain (Cosio & Lin, 2020).
Distraction
The brain has a limited capacity for attention, and there are only so many things it can concentrate on at the same time. Pain sensations compete for attention with all the other things going on. Just how much attention the brain gives each thing depends on a number of factors, including:
- How long the person has been dealing with pain
- Current mood
- Propensity to anxiety, rumination, and catastrophizing
Diverting attention (distracting) from feelings and thoughts of pain is a well-researched pain coping strategy. Mental distractions actually block pain signals from the body before they ever reach the brain. Distraction is shifting attention away from pain or painful stimuli to something more engaging or enjoyable. Research supports the use of distraction for acute pain among infants and children, with less consistent evidence for adolescents and adults (Stanford Health Care, 2021; Keane, 2021).
Mindfulness-Based Interventions
Mindfulness-based interventions (e.g., meditation) have been found to have significant effects on chronic pain, yet the mechanisms underlying these effects are not well understood.
The most widely used is mindfulness-based stress reduction (MBSR), which has been found to be effective in reducing the adverse impact of chronic pain. MBSR can increase the ability to tolerate or withstand distressing emotional or physical states and selectively alter the unpleasantness of pain. MBSR uses a combination of body awareness, mindfulness meditation, and movement to help people become focused on and accepting of the present moment (Cosio & Demyan, 2021).
Virtual Reality (VR)
Virtual reality therapies have been shown to effectively distract patients who suffer from chronic and acute pain. Virtual reality is a computer-generated world that simulates real-life experiences through senses and perception. An individual using VR equipment is able to look around the artificial world, move around in it, and interact with virtual features or even thoughts. This world is commonly created by using a VR headset that consists of a head-mounted display with a small screen positioned in front of the eyes, stereo sound, and sensors.
Virtual reality provides immersive experiences that absorb more of the brain’s attention. With fewer mental resources left to process pain signals, people perceive less pain. VR causes a reduction of the electrical signals through which neurons communicate. Further validation tests of EEG and investigation on VR effects are needed to better understand how our brain acts while immersed in a virtual world (VirtualTimes, 2021).
Virtual reality has also been shown to benefit children by reducing the fear and anxiety they experience before a procedure. Children tend to be more cooperative when engaged in VR, with less movement, less fear, and lower pain scores (Children’s Hospital Los Angeles, 2021).
Mirror Therapy (MT)
Mirror therapy is a rehabilitation therapy in which a mirror is placed between the arms or legs so that the image of a moving, nonaffected limb gives the illusion of normal movement in the affected limb. Mirror therapy exploits the brain’s preference to prioritize visual feedback over somatosensory/proprioceptive feedback concerning limb position. The reflection “tricks” the brain into thinking there are two healthy limbs.
Mirror therapy has been used to manage phantom leg or arm pain, reduce pain following a stroke, and for patients with a complex regional pain syndrome (Physiopedia, 2022d).
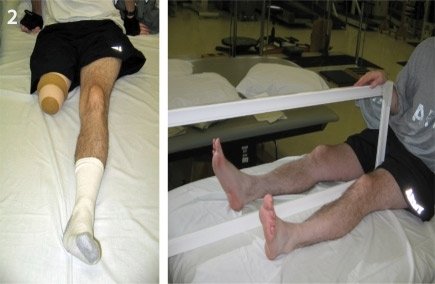
Using a mirror, the brain is “tricked” into seeing two limbs. (Source: © Sköld et al., 2011.)
Yoga
Yoga is a mind-body and exercise practice that helps relieve chronic pain. Yoga has many of the same benefits as mindfulness practice due to the common focus on breath, body, and present-moment awareness. There are different types of yoga, with the most evidence of benefit being shown through Iyengar yoga, hatha yoga, and Viniyoga.
It is not fully understood how yoga helps with pain, but emerging evidence suggests it might help people more effectively control how they think and feel, both mentally and physically. It may also work by improving muscle flexibility, promoting relaxation, reducing inflammation, or increasing the release of pain-relieving endorphins, as well as improving confidence and the sense of self-control. Most evidence for the use of yoga is in those with chronic back pain, arthritis, headaches/migraines, irritable bowel syndrome, fibromyalgia, and carpal tunnel syndrome (DHWA, 2021).
Tai Chi and Qigong
Tai chi and qigong are forms of traditional Chinese exercise that incorporate the concepts of two opposing forces—yin and yang. Both exercises are based on the idea and core principle that increasing energy in the body, known as chi, through gentle and repeated movements can enhance a person’s well-being. Pain or sickness is believed to occur when the flow of chi is blocked or when yin and yang energies are out of balance. The joints are seen as gates that control the flow of chi and that can be opened by using slow and gentle swaying movements, deep breathing, and mental focus.
The exact mechanism behind relief of chronic pain through the practice of qigong or tai chi isn’t fully known. Some researchers theorize that pain relief is achieved by eliminating muscular tension through deep relaxation or boosting endorphins. Others believe that the exercises may affect the autonomic nervous system, increasing parasympathetic tone, which is related to a relaxed state of body with a reduction in stress hormones (Marks, 2022; Winchester Hospital, 2022).
Evaluating the Effectiveness of Interventions
There are multiple outcome measures required to adequately assess the pain experience and how it has been modified by pain management interventions. The outcome of pain management is done by assessing:
- The degree of analgesic effect in comparison to the patient’s baseline
- The time to onset of the analgesic effect and the time to maximum reduction in pain intensity
- The duration of the analgesic effect
- Measures of physical functioning
- Measures of emotional functioning
- Secondary effects related to the treatment
(Edward, 2021)
THE JOINT COMMISSION PAIN MANAGEMENT STANDARDS
Because pain management is deemed essential to the provision of quality healthcare, many organizations have developed guidelines, standards, or principles by which professional practice is measured. The Joint Commission provides guidelines for accredited organizations in the leadership and provision of care, treatment and services, and assessment and management of pain. These guidelines state that the healthcare organization shall:
- Provide staff and licensed independent practitioners with educational programs and resources regarding pain management and the safe use of opioid medication
- Provide readily accessible, accurate information regarding opioid treatment programs that can be used for patient referrals
- Provide readily available screening and assessment tools and ensure they are used appropriately
- Develop and monitor performance-improvement activities specific to pain management and safe opioid prescribing
- Provide staff and licensed independent practitioners information on available services for consultation and referral of patients with complex pain management needs
- Provide nonpharmacologic pain treatment modalities relevant to its patient population and assessed needs of the patient
- Facilitate access to the Prescription Drug Monitoring Program (PDMP) databases
- Provide monitoring of postoperative patients on opiates and/or on opiates combined with other pain medications
- Educate the patient and family at discharge regarding the pain management plan; side effects of treatment; impact on activities of daily living; and safe use, storage, and disposal of opioids when prescribed
(TJC, 2021)
THE INTERDISCIPLINARY NATURE OF MANAGING PAIN
The most important member of the interdisciplinary team is the person with pain—the patient. Other team members can include:
- Significant others (family, friends, etc.)
- Physicians, physician assistants, and nurse practitioners
- Nurses
- Psychologists
- Physical therapists
- Occupational therapists
- Recreational therapists
- Vocational counselors
- Pharmacists
- Nutritionists/dietitians
- Social workers
- Support staff
- Volunteers
Role of the Nurse
Many disciplines are involved in managing a patient’s pain, and nurses play a pivotal role in the assessment, monitoring, interpretation, and evaluation of pain. Effective pain management by nurses is fundamental to quality of care and is the first responsibility of the nurse.
NURSING STANDARDS OF CARE
Standards of care for nurses in the management of pain include, but are not limited to:
- Acknowledging and accepting the patient’s pain
- Identifying the most likely source of the pain
- Assessing pain at regular intervals, including each new complaint of pain, utilizing a pain assessment tool
- Assessing barriers to effective pain management
- Reporting the patient’s level of pain and developing a plan of care that includes interdisciplinary input
- Aggressively treating side effects such as nausea, vomiting, constipation, etc.
- Educating patient, family, and significant others on:
- Their role in the pain management plan and expected outcomes
- Detrimental effects of unrelieved pain
- Overcoming barriers to effective pain management
- Evaluating effectiveness of strategies and nursing interventions
- Documenting and reporting the interventions, patient responses, outcomes
- Advocating for the patient and family for effective pain management
(MD BON, n.d.)
PATIENT EDUCATION AND OPIOID MEDICATIONS
Nurses have an important role to play in educating patients about the proper use of opioid medications. The following information should be included in the education provided to patients who are receiving opioids as well as to their caregivers:
- Take opioids as prescribed.
- In case of a missed dose or if the pain is not managed by the recommended doses:
- If pain is not managed, talk with the healthcare team and do not take extra doses.
- If a dose is missed, take it as soon as possible; however, if it is almost time for the next dose, skip the missed dose and go back on schedule.
- Do not double doses.
- For slow-release medication, if more than four hours late, do not take it.
- Oral capsules may be opened and mixed with cool foods, but extended-release medication should not be opened, crushed, broken, or chewed.
- It can be dangerous to use CNS depressants, including sedatives, alcohol, or illicit drugs, when taking opioid medications.
- Due to the addictive nature of opioids, discontinuation should be accomplished by tapering the drug’s dose with the assistance of one’s primary care provider.
- Opioids slow peristalsis and can lead to constipation; adequate fluid and fiber intake will facilitate the passage of stool.
- Opioids can impact one’s ability to drive or operate machinery and affect one’s balance, increasing the risk for falls.
- Death due to respiratory depression is a potential side effect.
- Drugs should never be shared.
- To avoid diversion, opioids should be locked in a secure location.
- Unused opioids should be disposed of safely (see table below).
(Jackson, 2020)
| Drug Name | Examples |
|---|---|
| (U.S. FDA, 2020) | |
| Drugs That Contain Opioids | |
| Any drug that contains the word buprenorphine | Belbuca, Buavail, Butrans, Suboxone, Subutex, Zubsolv |
| Any drug that contains the word fentanyl | Abstral, Actiq, Duragesic, Fentora, Onsolis |
| Any drug that contains the words hydrocodone or benzhydrocodone | Apadaz, Hysingla ER, Norco, Reprexain, Vicodin, Vicodin ES, Vicodin HP, Vicoprofen, Zohydro ER |
| Any drug that contains the word hydromorphone | Exalgo |
| Any drug that contains the word meperidine | Demerol |
| Any drug that contains the word methadone | Dolophine, Methadose |
| Any drug that contains the word morphine | Arymo Er, Avinza, Embeda, Kadian, Morphabond ER, MS Contin, Oramorph SR |
| Any drug that contains the word oxycodone | Codoxy, Combunox, Oxadydo (formerly Oxecta), Oxycet, Oxycontin, Percocet, Percodan, Roxicet, Roxicodone, Roxilox, Roxybond, Targiniq ER, Troxyca ER, Tylox, Xartemis XR, Xtampza ER |
| Any drug that contains the word oxymorphone | Opana, Opana ER |
| Any drug that contains the word tapentadol | Nucynta, Nucynta ER |
| Drugs That Do Not Contain Opioids | |
| Any drug that contains the terms sodium oxybate or sodium oxybates | Xyrem, Xywav |
| Diazepam rectal gel | Diastat, Diastat Acudial |
| Methylphenidate transdermal system | Daytrana |
ANA POSITION STATEMENT ON PAIN MANAGEMENT
The American Nurses Association Position Statement (2018) on the nurse’s role and ethical responsibility in the management of pain states that:
- Nurses have an ethical responsibility to provide clinically excellent care to relieve pain. Clinically excellent pain management considers clinical indications, mutual identification of goals for pain management, and ongoing reassessment with the patient of the effectiveness of pain control efforts.
- Nurses use the nursing process to guide actions to improve pain management and should ensure that each patient experiencing pain has an individualized pain management plan with appropriate monitoring to avoid undertreatment, overtreatment, or addiction.
- Nurses provide respectful, individualized nursing interventions to all patients experiencing pain regardless of the person’s personal characteristics, values, or beliefs.
- Nurses consider that multimodal and interprofessional approaches are necessary to achieve effective pain relief.
- Nurses use pain management modalities that are evidence-based. Lack of knowledge and understanding of best practices for assessing and optimally managing pain constrain the nurse’s ability to effectively minimize pain.
- Nurses have an obligation to assess and address factors within themselves and their practice environments that constrain ability and willingness to relieve pain and the suffering it causes.
- Nurses advocate for policies to assure access to all effective modalities. Nurses have a duty to advocate for improved parity in coverage for all effective pain relief modalities
Role of the Occupational Therapist
The role of the occupational therapist within an integrative pain management program focuses on function in daily living and takes a holistic and comprehensive approach to evaluate structural, physiologic, psychological, environmental, and personal factors that influence the experience of pain. The information obtained by patient evaluation is then used in the application of self-management strategies, functional activities, hands-on techniques, and specific exercises to improve function and participation.
OCCUPATIONAL THERAPY INTERVENTIONS
Depending on the area impacted by chronic pain, the occupational therapist provides the following interventions:
Physical mobility:
- Adaptive equipment selection and training
- Positioning equipment and strategies
- Functional mobility training (e.g., static positioning, dynamic movement, transfers, lifting and bending techniques)
Activities of daily living/self-care:
- Neuromuscular re-education
- Nerve mobilization
- Functional range of motion and strengthening exercises
- Activity pacing and energy conservation strategies
- Ergonomic and body mechanics training
- Fall prevention and safety
- Home evaluation
Instrumental activities of daily living:
- Adaptive equipment selection and training
- Transportation training, including comprehensive driver evaluations and driver rehabilitation
Health management:
- Patient education and disease self-management training, including trigger identification, symptom tracking, and pain flare-up planning
- Pain coping strategies, including physical modalities, complementary and alternative pain coping strategies, sensory strategies, self-regulation, and mobilization
- Pain and assertive communication training
- Medication management
- Eating routine strategies to avoid dietary pain triggers and improve energy management
- Establishing sustainable physical activities
- Time management strategies
Rest and sleep:
- Sleep hygiene and positioning strategies
- Cognitive behavioral therapy for insomnia
- Energy conservation and fatigue management
Education and work:
- Academic and work accommodations
- Ergonomic and body mechanics training
- Sensory strategies to monitor environmental triggers or exacerbating factors
- Advocacy and self-advocacy training
- Assertive communication training
- Community reintegration, including gradual re-entry plans
- Activity pacing and energy
- Environmental modifications
- Community and online resources exploration
- Compensatory cognitive strategies
Play, leisure, and social participation:
- Strategies to prevent social isolation
- Assertive communication strategies
- Personal values and interests exploration
(Reeves et al., 2022)
Role of the Physical Therapist
Physical therapy is one of the most important nonpharmacologic measures to be considered in the management of pain. The physical therapist evaluates the patient with pain in order to:
- Determine the identity of the pain mechanism(s) to guide treatment
- Identify physical and psychosocial factors impacting pain so they can be addressed
- Assess the impact of pain on physical and psychosocial function
- Select appropriate goals
- Determine whether the patient requires referral to other healthcare providers
The goals of physical therapy include the reduction of pain, restoration of function, improved mobility, prevention or limitation of permanent physical disabilities, and encouragement of self-management through the use of physical, cognitive, and behavioral approaches to help reduce the impact of pain and disability (Sullivan et al., 2019).
PHYSICAL THERAPY INTERVENTIONS
When physical therapists work with patients who are experiencing pain, tests and measures are utilized to determine the causes of pain and to assess its intensity, quality, physical characteristics, and progression. Patients are also evaluated for risk factors for pain in order to prevent future pain issues. These factors may include disease history, cognitive and psychological factors, negative beliefs, and sedentary lifestyle.
Once contributors to the pain are identified, the therapist works with the patient to design an evidence-based management program with goals that are specific, measurable, achievable, relevant, and time-framed.
The physical therapist then implements the management program, which includes active approaches and passive approaches as indicated. These approaches include:
- Education about pain and how to manage pain, working with the patient toward regaining the ability to perform normal activities of daily living.
- Strengthening and flexibility exercises to improve movement with less pain. A graded exercise program may be instituted that gradually increases according to abilities. Exercises help to improve movement and coordination, reduce stress and strain on the body, and decrease pain.
- Manual therapies using hands-on techniques to manipulate or mobilize tight joint structures and soft tissues. Manual therapy may help increase range of motion, improve tissue quality, and reduce pain. Such therapies may include peripheral joint mobilization, myofascial mobilization, spinal mobilization, soft tissue mobilization, and therapeutic massage.
- Instruction in proper postural awareness and body mechanics, in order to help patients use their body more efficiently.
- Physical agents, which may include electrotherapies.
The therapist educates and supports the patient to adopt active rather than solely passive pain-management strategies that are meaningful to the patient and achievable, using motivational strategies and adherence techniques to support compliance.
Physical therapists include cognitive and behavioral approaches that support improved functional movement and pain outcomes, along with self-management strategies, as a key component of the management plan (IASP, 2021c).
MODALITIES
Modalities physical therapists may employ in pain management include:
- Thermotherapy
- Dry heat
- Hot packs
- Paraffin baths
- Tecar therapy
- Cryotherapy
- Ice packs
- Ice spray
- Immersion
- Ice massage
- Cryokinetics
- Biofeedback
- Manual therapies
- Massage
- Connective tissue massage
- Therapeutic massage
- Manipulation/mobilization
- Dry needling
- Soft tissue mobilization
- Spinal and peripheral joint mobilization
- Neural tissue mobilization
- Passive range of motion
- Electric stimulation
- Electric stimulation for tissue repair (ESTR)
- Functional electrical stimulation (FES)
- High-voltage pulsed current (HVPC)
- Neuromuscular electrical stimulation (NMES)
- Transcutaneous electrical nerve stimulation (TENS)
- Electrotherapeutic delivery of medications
- Iontophoresis
- Hydrotherapy
- Contrast bath
- Pools
- Pulsatile lavage
- Whirlpool tanks
- Acoustic
- Ultrasound
- Phonophoresis
- Traction devices
- Intermittent
- Positional
- Sustained
- Light therapy
- Laser (low level and high power)
- Ultraviolet
- Infrared and near infrared
- Cold laser therapy
PHYSICAL THERAPY AND THE OPIOID EPIDEMIC
The American Physical Therapy Association, along with others, developed the NQP Playbook: Opioid Stewardship to provide concrete strategies and implementation examples for effective pain management and opioid stewardship. Other programs include the APTA’s #ChoosePT opioid awareness campaign, which encourages consumers and prescribers to follow the CDC’s opioid-prescription guidelines (APTA, 2021).
OPIOID MISUSE, ABUSE, AND DIVERSION
Along with attempts to improve identification and treatment of pain, there has been an equal rise in prescription opioid addiction and abuse in the United States. Opioid misuse, abuse, and diversion are major problems with serious consequences.
In the past, opioids were often not prescribed for a patient because of the fear of addiction. In the 1980s, the opioid epidemic in the United States arose through an assemblage of well-intentioned efforts to improve pain management by doctors and through aggressive marketing by pharmaceutical manufacturers. States began to pass intractable pain treatment acts, which removed threat of prosecution for physicians who treated their patients aggressively with a controlled substance. In 1995, the American Pain Society began a campaign that framed pain as a “fifth vital sign” that should be monitored and managed in the same way as heart rate and blood pressure (DeWeerdt, 2019).
Now practitioners have returned to the fear of addiction and become, once again, reluctant to prescribe opioids. Clearly, there is a dilemma between the need to address opioid abuse and overdose while continuing to ensure people with pain receive safe, effective treatment.
Scope of the Problem
The National Institute on Drug Abuse (2022a) reports that:
- Among people ages 12 and older in 2020, an estimated 2.3 million people in the United States had a prescription opioid use disorder in the past year.
- Nearly 92,000 persons in the United States died from drug-involved overdose in 2020 due to illicit drugs and prescription opioids. The national overdose deaths involving prescription opioids among all ages in 2020 was 16,416.
- Among young people in 2021, an estimated 4.4% of 12th graders reported misusing any prescription drug in the past 12 months.
- 50,000 individuals used heroin for the first time, and 14,480 deaths from heroin occurred in 2020.
National Survey on Drug Use and Health (2020) reported that the state of Oregon ranked:
- First in the nation for illicit drug use disorder
- First in the nation for opioid misuse
- First in the nation for methamphetamine use
- Fifth in the nation for alcohol disorder
Oregon also has the worst ranking (50th) in the nation for people needing but not receiving treatment for substance use disorders.
Multiple variables contribute to the opioid crisis including:
- Geographic conditions
- Treatment accessibility
- Medication disposal services
- Workplace environment
- Prescribers’ perception of risk
- Overprescription of opioids or undertreatment of pain
- Types of prescription opioid formulation available
- Community norms
- Access to legal and illegal opioids
(Jalali et al., 2020)
Drug diversion can be defined as any act or deviation that removes a prescription drug from its intended path from the manufacturer to the patient and can occur anywhere along the continuum: manufacturer, wholesale distributor, retail pharmacy, hospitals and other healthcare organizations, prescribers, healthcare professionals who administer the medication, or the patient for whom the medication is prescribed (ASHP, 2022).
The effort to prevent misuse, abuse, and diversion involves government and regulatory agencies, drug researchers and manufacturers, as well as healthcare institutions and individual clinicians.
CDC Guidelines for Prescribing Opioids
In 2022, the CDC updated its guidelines for prescribing opioids for the treatment of pain. Whereas the 2016 guideline focused on recommendations for primary care physicians, the newer guideline expands the scope to additional clinicians whose scope of practice includes prescribing opioids (e.g., physicians, nurse practitioners and other advanced-practice registered nurses, physician assistants, and oral health practitioners). The 2022 guidelines address four main issues, including:
- Making a determination about whether or not to initiate opioids for pain
- Selecting the appropriate opioid and determining the dosage
- Deciding the duration of the initial opioid prescription and conducting follow-up
- Assessing the risk and addressing the potential harms of opioid use with the patient
The recommendations in the 2022 guidelines aim to improve communication between clinicians and patients about the risks and effectiveness of pain treatment; improve pain, function, and quality of life for persons with pain; and reduce the risks associated with opioid pain treatment (including opioid use disorder, overdose, and death) as well as with other pain treatment.
The practice guidelines include 12 recommendations for clinicians who are prescribing opioids for outpatients ages 18 years and older with pain that is acute (duration of <1 month), subacute (duration of 1–3 months), or chronic (duration of >3 months), excluding pain management related to sickle cell disease, cancer-related pain treatment, palliative care, and end-of-life care.
- Nonopioid therapies are at least as effective as opioids for many common types of pain. Maximize the use of nonpharmacologic and nonopioid pharmacologic therapies appropriate for the condition and the patient, and only consider opioid therapy for acute pain if benefits are expected to outweigh risks to the patient. Discuss benefits and risks with the patient prior to prescribing opioid therapy.
- Nonopioid therapies are preferred for subacute and chronic pain. Maximize use of nonpharmacologic and nonopioid pharmacologic therapies as appropriate for the specific condition and patient. Consider opioid therapy if expected benefits are anticipated to outweigh risks, and work with the patient to establish treatment goals for pain and function. Consider how opioid therapy will be discontinued if benefits do not outweigh risks.
- When starting opioid therapy for acute, subacute, or chronic pain, prescribe immediate-release opioids instead of extended-release and long-acting (ER/LA) opioids.
- When opioids are initiated for opioid-naive patients with acute, subacute, or chronic pain, prescribe the lowest effective dosage. If opioids are continued for subacute or chronic pain, prescribe the lowest effective dosage. Avoid increasing dosage above levels likely to yield diminishing returns in benefits relative to risks.
- For those patients already receiving opioid therapy, carefully weigh benefits and risks and exercise care when changing opioid dosages. Work closely with patients to optimize nonopioid therapies while continuing opioid therapy. If benefits do not outweigh risk of continued opioid therapy, optimize other therapies and work closely with patients to gradually taper to lower dosages, or appropriately taper and discontinue opioids. Unless there are indications of a life-threatening issue such as warning signs of impending overdose (e.g., confusion, sedation, slurred speech), opioid therapy should not be discontinued abruptly, and clinicians should not rapidly reduce opioid dosages from higher dosages.
- When opioids are needed for acute pain, prescribe no greater quantity than needed for the expected duration of pain severe enough to require opioids.
- Evaluate benefits and risks with patients within 1–4 weeks of starting opioid therapy for subacute or chronic pain or of dosage escalation. Regularly re-evaluate benefits and risks of continued opioid therapy with patients.
- Before starting and periodically during continuation of opioid therapy, evaluate risks for opioid-related harms and discuss risks with patients. Work with patients to incorporate into the management plan strategies to mitigate risk, including offering naloxone.
- When prescribing initial opioid therapy for acute, subacute, or chronic pain, and periodically during opioid therapy for chronic pain, review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program data to determine whether the patient is receiving opioid dosages or combinations that put the patient at high risk for overdose.
- When prescribing opioids for subacute or chronic pain, consider the benefits and risks of toxicology testing to assess for prescribed medications as well as other prescribed and nonprescribed controlled substances.
- Use particular caution when prescribing opioid pain medication and benzodiazepines concurrently and consider whether benefits outweigh risks of concurrent prescribing of opioids and other central nervous system depressants.
- Offer or arrange treatment with evidence-based medications for patients with opioid use disorder. Detoxification on its own, without medications for opioid use disorder, is not recommended because of increased risks for resuming drug use, overdose, and overdose death.
(Dowell et al., 2022)
OREGON PRESCRIPTION MONITORING PROGRAM (PDMP)
Oregon has developed a Prescription Drug Monitoring Program, and as of July 1, 2018, all prescribers in the state of Oregon were mandated to enroll in the program. The PDMP aids healthcare providers and pharmacists in managing patient prescriptions to improve quality of care and to support the appropriate use of prescription drugs. The program:
- Provides safer and more effective pain management for persons with chronic pain and limits risky opioid prescribing
- Supports medically necessary use of controlled substances
- Facilitates coordination of care among providers
- Aids in the identification of potential misuse, abuse, or diversion of prescription drugs
- Assists in initiating compassionate conversations
- Helps with intervention and treatment of people with substance use disorder
(OSS, 2018)
Abuse-Deterrent Opioids
In an attempt to respond to the abuse of opioid medications, abuse-deterrent products are being formulated and approved for use by the FDA. Abuse-deterrent drugs have been shown to meaningfully discourage use and deter abuse. However, these medications do not obstruct the use of opioids and do not prevent abuse. The science of abuse deterrence is quite new and rapidly evolving.
Abuse-deterrent formulations can be classified as a physical/chemical barrier that prevents drug release following manipulation of the drug or changes the physical form of the drug using chemicals that render it less amenable to abuse.
Agonist/antagonist combinations interfere with, reduce, or defeat the euphoria associated with abuse. The antagonist can be sequestered and released only when the product is manipulated. It is not clinically active when the drug is swallowed but becomes active when it is injected or snorted.
An aversion type of abuse-deterrent drug has a substance added that produces an unpleasant effect if the drug is manipulated or taken at a higher dosage than directed. It can include a substance that irritates the nasal mucosa if ground and snorted.
Delivery system methods can also offer resistance to abuse. Sustained-release depot injectable or subcutaneous implant formulations may be difficult to manipulate.
Other drugs may be classified as combinations in which two or more of the above methods could be combined to deter abuse.
Opioids with FDA-approved labeling describing abuse-deterrent properties include:
- Oxycontin
- Hysingla ER
- Xtampza ER
- RoyBond
Generic opioids with FDA-approved labeling describing abuse-deterrent properties include:
- Hydrocodone bitartrate
(U.S. FDA, 2021)
Management of Opioid Overdose
It is important to consider opiate overdose or toxicity in a lethargic patient with no other identifiable cause. Care of the patient at the scene depends on the vital signs. If the patient is comatose and in respiratory distress, airway control must be obtained prior to any other action. Endotracheal intubation is highly recommended for all patients unable to protect their airways.
If there is a suspicion of opiate overdose, naloxone is administered to reverse respiratory depression. Naloxone, an opioid antagonist, rapidly reverses an opioid overdose. One should be aware that naloxone can also cause agitation and aggression when it reverses the opiate.
If the patient is a known drug abuser, the lowest dose of naloxone to reverse respiratory distress should be administered. In the ambulance, the patient may become combative or violent, and use of restraints may be required. If the individual has no intravenous access, naloxone can be administered intramuscularly, intranasally, intraosseous, or via the endotracheal tube. Data show that the intranasal route is as effective as the intramuscular route in the prehospital setting (Schiller et al., 2022).
Clinician Efforts to Reduce Opioid Abuse
There are a number of validated screening tools available for use to aid clinicians in early detection of problematic substance use. The Screener and Opioid Assessment for Patients with Pain (SOAPP-R) is one of several such tools used for screening, brief intervention, and referral to treatment.
An informed consent should be considered for patients who are beginning long-term therapy with opioid analgesics to help ensure they understand the side effects, risks, conditions, and purpose of their treatment. This document can clearly define treatment expectations and resolve any questions or concerns patients may have before treatment is initiated.
Clinicians frequently monitor patients in person to assess for and document benefits and harms of treatment, including concerning behaviors that may indicate misuse or a use disorder. Guidelines vary, but visits should generally occur at least every three months and more frequently in higher risk circumstances, such as during periods of dose adjustment.
Urine testing is commonly performed prior to and during chronic opioid therapy for patients with chronic pain. Scheduled or random urine drug testing (with informed consent) is often part of the opioid agreement.
Patients receiving long-term opioid therapy are monitored for the three components of addiction:
- Loss of control
- Craving and preoccupation with use
- Use despite negative consequence
Clinicians must be aware of indications of opioid use disorder, which include:
- Inconsistent healthcare use patterns
- Missed appointments
- Lack of engagement with nonmedication treatments
- Lack of follow-through with recommendations
- Illicit drug use
- Problematic medication (e.g., escalating doses, early refills)
- Family concerns about use
- Decreased function and loss of roles
- Extreme difficulty with even a slow opioid taper
- Signs/symptoms of drug use (e.g., intoxication, overdose, track marks)
Should the clinician determine substance use disorder may exist, the patient is provided with information about local inpatient detoxification services, methadone maintenance programs, or buprenorphine treatment.
It is important that clinicians recognize when to taper and/or transition a patient off of opioid-based medications and document why opioid treatment can no longer be prescribed (NIDA, 2020b; Mahajan, 2021; Becker & Starrels, 2021).
IDENTIFYING DRUG-SEEKING PATIENTS
Most patients who complain of pain are honestly seeking relief from discomfort. Others seek drugs in order to cope with addiction or to provide income. Differentiating between the two can be very difficult.
Drug seekers include people of every age, gender, and socioeconomic status. Often these people initially used prescription drugs for valid medical conditions, and drug-seeking behaviors may have developed as a result of disease progression, undertreatment of pain, tolerance to the medication, or unrecognized addiction. Only a small number of drug seekers do so to divert opioids for illicit sale.
There are some common characteristics that can provide clues regarding the nature of a patient’s intent. The patient who is drug seeking may:
- Come from a location that is far away, perhaps across state lines
- Have seen many doctors in a short period of time
- Present with specific complaints that are often subjective (back pain, headache)
- Bring old medical records they have been carrying around to many different doctors to get a pain prescription
- Use multiple pharmacies
- Claim an allergy to all pain medications except the one they are seeking as well as to diagnostic test contrast medium to avoid tests
- Suggest the medication, dose, and quantity being sought
- Be unwilling to consider any other treatments and does not want to listen to anything the clinician has to say
- Call or show up requesting a prescription at off hours, when the office is closing or right before the weekend/holiday when it is less likely their usual care provider(s) can be reached
- Lie or their story does not make sense (it is imperative to take a detailed history to look for inconsistencies in a made-up story)
- Exaggerate symptoms, with inconsistent behavior from waiting room to treatment room
- Become aggressive when different medications are suggested
- Give false information, such as a fake address or a disconnected phone number
- Be on multiple controlled substances, such as opioids and benzodiazepines
- Be excessively talkative, friendly, or helpful
However, drug-seeking patients with addictions are not the only ones who may engage in these behaviors. Over time, patients with true chronic pain can elicit some of these same behaviors (Girgis, 2021).
ADDRESSING DRUG-SEEKING BEHAVIORS
There are a number of strategies healthcare providers can utilize in the management of individuals with drug-seeking behaviors. The following are suggestions made by medical risk management advisors:
- Perform a complete review of the patient’s pertinent history, and conduct a thorough medical evaluation, addressing and documenting all objective signs and symptoms of pain.
- Exercise concern when dealing with patients who are not interested in having a physical examination, are unwilling to authorize release of prior medical records, or have no interest in a diagnosis or a referral.
- Be cautious if a new patient has an unusual knowledge of controlled substances or requests a specific controlled substance and is unwilling to try any other medication.
- Utilize the state prescription monitoring program (PDMP) to identify patients at risk for drug diversion and/or “doctor shopping.”
- Implement a systematic procedure for refilling prescriptions and educating appropriate staff regarding the policy.
- Inform patients verbally and in writing about the medication refill procedure.
- Establish a treatment agreement with the patient that outlines the provider’s expectations, which should address:
- Number and frequency of prescription refill
- Early refills
- Replacement of lost or stolen medications
- Specific reasons for discontinuing or changing the drug therapy
- Consider referral to or consultation with a pain management specialist for patients not responding to the treatment plan.
- Exercise the right to terminate a patient who fails to follow the treatment plan or adhere to the treatment agreement.
(Jakucs, 2021; Johnson, 2017)
Confronting patients believed to be seeking drugs can be difficult. Confrontation may turn out to be therapeutic, but it can also be dangerous. It is best to avoid confronting a drug-seeking patient alone. The clinician should consider involving psychiatric support, social service assistance, facility security, and in some instances, local law enforcement.
PAIN MANAGEMENT AGREEMENT
A pain management agreement documents the understanding between a prescriber and a patient regarding prescribed medications being taken for pain management. Its purpose is to prevent misunderstandings about certain medications and to help the prescriber and patient comply with laws regarding controlled substances. A typical pain management agreement:
- Requires the patient to use one pharmacy only for all prescription refills
- Identifies expected benefits of medications and the risk associated with their misuse
- Lists the possible side effects that can occur
- Requires notification when the same or similar medication is prescribed by other healthcare providers
- Lists the conditions for issuing refills or replacement prescriptions
- Requires regular evaluations of pain
- Requires random screenings for misuse of medication
- Describes the conditions under which therapy can be changed or discontinued
Drug Diversion and Addiction among Healthcare Professionals
Because healthcare professionals are trusted with others’ health and well-being, they are not often suspected of drug addiction themselves; however, they are just as likely as anyone else to become addicted and are at a higher risk for addictive behaviors involving opioids because of their increased access to them.
ADDRESSING DRUG DIVERSION IN HEALTHCARE WORKERS
It is a legal and ethical responsibility for healthcare professionals to uphold the law and to help protect society from drug abuse, and it is a professional responsibility to prescribe and dispense controlled substances appropriately, guarding against abuse while ensuring that patients have medication available when it is needed. Each healthcare professional also has a personal responsibility to protect their practice from becoming an easy target for drug diversion and must be aware of the potential situations where it can occur and the safeguards that can be utilized to prevent such diversion.
Healthcare professionals often avoid dealing with drug impairment in their colleagues. It is natural to be reluctant about approaching such colleagues for fear of their anger since it may result in retribution. Another concern is fear for the colleague’s loss of professional practice. As a result, employers or coworkers can become enablers of those colleagues whose professional competence is being impaired by drug abuse, and thereby are being protected from the consequences of their behavior. Some enabling behaviors include:
- Ignoring poor performance
- Lightening or changing the colleague’s patient assignment
- Accepting excuses
- Allowing oneself to be manipulated
- Being fearful of confronting a colleague if patient safety is in jeopardy
When the signs and symptoms of drug abuse are evident in a colleague, it is time to become concerned and involved, taking the following steps:
- Check the agency’s written drug and alcohol policy and follow recommendations.
- Document suspicions regarding the colleague, including any complaints, concerns, behavior patterns, or witnesses to behaviors.
- Bring concerns to management.
If it is known that drugs are being sold or stolen, it is important not to intervene alone and to contact security or notify the police. If a DEA registrant becomes aware of a theft or significant loss involving controlled substances, it must also immediately be reported to the nearest DEA office, the healthcare worker’s state licensing board, as well as the local police department (U.S. DEA, 2019).
INDICATORS OF DRUG ADDICTION IN THE WORKPLACE
The following signs and symptoms may indicate a drug-related problem in a healthcare professional:
- Recurring absences or tardiness
- Frequent disappearances from the work site, such as long trips to the bathroom or stockroom where drugs are kept
- Excessive amounts of time spent near a drug supply
- Volunteering for overtime and being at work when not scheduled to be there
- Unreliability in keeping appointments and meeting deadlines
- Work performance that alternates between high and low productivity
- Mistakes made due to inattention, poor judgment, and bad decisions
- Confusion, memory loss, and difficulty concentrating or recalling details and instructions
- Ordinary tasks requiring greater effort and consuming more time
- Deterioration in interpersonal relations with colleagues, staff, and patients
- Rarely admitting errors or accepting blame for errors or oversights
- Sloppy recordkeeping, suspect ledger entries, and drug shortages
- Inappropriate prescriptions for large narcotic doses
- Insistence on personally administering injectable narcotics to patients
- Progressive deterioration in personal appearance and hygiene
- Uncharacteristic deterioration of handwriting and charting
- Wearing long sleeves when inappropriate
- Personality changes, mood swings, anxiety, depression, lack of impulse control
- Suicidal thoughts or gestures
- Patient and staff complaints about healthcare provider’s changing attitude/behavior
- Increasing personal and professional isolation
(U.S. DEA, 2019)
DEA RED FLAGS FOR DRUG DIVERSION
Prescribers
- Cash-only patients and/or no acceptance of worker’s compensation or private insurance
- Prescribing of the same combination of highly abused drugs
- Prescribing the same, typically high, quantities of pain drugs to most or every patient
- High number of prescriptions issued per day
- Out-of-area patient population
Dispensers
- Dispensing a high ratio of controlled to noncontrolled drugs
- Dispensing high volumes of controlled substances generally
- Dispensing the same drugs and quantities prescribed by the same prescriber
- Dispensing to out-of-area or out-of-state patients
- Dispensing to multiple patients with the same last name or address
- Sequential prescription numbers for highly diverted drugs from the same prescriber
- Dispensing for patients of controlled substances from multiple practitioners
- Dispensing for patients seeking early prescription refills
(WVPMP, 2019)
Addressing Pain in Individuals with Substance Use Disorders (SUDs)
Opioid use for pain management for patients with a history of SUDs may be considered if their use is carefully managed. This involves selecting the appropriate opioid, dosage titration, treatment agreements, and testing and monitoring.
When choosing the appropriate opioid, providers should select a medication that is safe and start with a low dose to ease pain, then titrate as needed to maintain pain relief without decreasing function or risking addiction or relapse.
When an effective dose has been determined, total opioid dose is increased slowly and only if needed, as tolerance develops. When monitoring for dosage, providers must be aware of both tolerance and hyperalgesia concerns. Tolerance can occur regardless of opioid type, dosage, route of administration and dosage schedule. Clinicians should be aware that hyperalgesia, or oversensitivity to pain, can occur in some patients using opioids for chronic pain.
When patients develop tolerance to the analgesic effects of a particular opioid, providers can consider either escalating the dosage or switching from one opioid to another, at a low dose that will effectively relieve pain without increasing the risk of relapse.
Before initiating opioid treatment, providers should determine whether the patient has access to a naloxone kit and prescribe one if they do not. (See also “Management of Opioid Overdose” earlier in this course.)
When pain has been resolved, the provider should gradually discontinue opioid therapy. Other reasons for discontinuing opioid treatment include:
- Opioids are no longer effective
- Adverse effects are unmanageable
- The patient does not adhere to the treatment agreement
- The patient is misusing or diverting the medication
If the reason is due to nonadherence to the treatment agreement or misuse of opioids, the patient should be referred for addiction treatment (SAMHSA, 2021).
ASSESSING RISK FOR DEVELOPING SUBSTANCE USE DISORDERS
Before introducing any opioids into a patient’s treatment regimen, an assessment is done to determine the patient’s risk for developing a substance abuse disorder (SUD).
Screening tools available to clinicians include:
- Opioid Risk Tool (ORT)
- Drug Abuse Screen Test (DAST-10 and DAST-20 for adolescents)
- Screener and Opioid Assessment for Patient with Pain-Revised (SOAPP-R)
- Brief Screener for Alcohol, Tobacco, and other Drugs (BSTAD)
These tools, however, commonly result in inaccurate findings and misinterpretations. For instance, since screening tools often rely on a patient’s self-report, a patient may falsify responses on questionnaires to avoid detection as a high-risk patient.
Other recommendations include drug testing, primarily urine screening. Drug testing offers a critical adjunct to clinical assessment of SUD risk. However, due to the ease with which samples can be adulterated, providers must carefully review their collection protocols and sample validation procedures to ensure optimal accuracy, which may require observed collection (NIDA, 2022b; Rosenquist, 2022).
CONCLUSION
Pain is a universal human experience, the strongest motivator for an individual to seek medical care, and one of the body’s most important protective mechanisms. For the past several decades researchers have been hard at work discovering exactly what pain is and how to prevent it or alleviate it.
It is imperative that healthcare professionals understand their role in managing pain as one of their primary obligations and responsibilities. It is the duty of all involved in caring for patients in pain to do everything possible to bring them relief. To do less is to fail to provide quality patient care. In order to best carry out this responsibility, it is necessary for all professionals to continue to expand their knowledge and skills in managing this crucial healthcare issue.
RESOURCES
American Society for Pain Management Nursing
CDC Clinical Practice Guideline for Prescribing Opioids for Pain
Guidelines on the management of chronic pain in children (WHO)
National Institutes of Health Pain Consortium
Oregon Medical Marijuana Program
Oregon Pain Management Module (Oregon Pain Management Commission)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Agranoff AB. (2020). Electrodiagnosis. Medscape. https://emedicine.medscape.com
Ahn A. (2020). Acupuncture. UpToDate. https://www.uptodate.com
American Massage Therapy Association (AMTA). (2022). Types of massage. https://www.amtamassage.org
American Nurses Association (ANA). (2018). Position statement: The ethical responsibility to manage pain and the suffering it causes. https://www.nursingworld.org
American Psychological Association (APA). (2022). Addictions. https://www.apa.org
American Physical Therapy Association (APTA). (2021). Beyond opioids: How physical therapy transforms pain management to improve health: 2021. https://www.apta.org
American Society of Health-System Pharmacists (ASHP). (2022). Controlled substances drug diversion pharmacy technician toolkit. https://www.ashp.org
Americans for Safe Access (ASA). (2021). 2021 State of the states report: An analysis of medical cannabis access in the United States. https://assets.nationbuilder.com
Anand K. (2022). Assessment of neonatal pain. UpToDate. https://www.uptodate.com
Anesthesia Key. (2019). Physical examination of the patient with pain. https://aneskey.com
Asher A. (2022). Blood tests for diagnosing back pain. WebMD. https://www.webmd.com
Ballantyne J, Fishman S, & Rathmell J (Eds.). (2019). Bonica’s management of pain (5th ed.). Wolters Kluwer.
Bar-Shalita T & Cermak S. (2020). Multi-sensory responsiveness and personality traits predict daily pain sensitivity. Front. Integr. Neurosci., 13, 77. http://doi.org/10.3389/fnint.2019.00077
Beaumont Health. (2022). Types of pain. https://www.beaumont.org
Becker W & Starrels J. (2021). Prescription drug misuse: Epidemiology, prevention, identification, and management. UpToDate. https://www.uptodate.com
Brennan D. (2021). What to know about ultrasound physical therapy. WebMD. https://www.webmed.com
Bukstein O. (2022). Approach to treating substance use disorder in adolescents. UpToDate. https://www.uptodate.com
Buowari DY. (2021). Pain management in older persons. In Update in geriatrics. IntechOpen. https://doi.org/10.5772/intechopen.93940
Canadian Association of Schools of Nursing/Association of Faculties of Pharmacy of Canada (CASN/AFPC). (2021). Module 7: Pain management—topic E. https://ououd.ca
Caring to the End. (2022). Pain assessment. http://www.ca
Casannova-Perez R, Apodaca C, Bascom E, Mohanraj D, et al. (2022). Broken down by bias: Healthcare biases experienced by BIPOC and LBGTQ+ patients. AMIA Snnu Symp Proc, 21, 275–84.
Children’s Hospital Los Angeles. (2021). A game changer: Virtual reality reduces pain and anxiety in children. Science Daily. https://www.sciencedaily.com
Cleveland Clinic. (2022). Pain: Psychogenic pain. https://my.clevelandclinic.org
Comprehensive MedPsych Systems. (2021). Pain. https://www.medpsych.net
Cosio D & Demyan A. (2021). Applying mindfulness-based stress reduction for comorbid pain and PTSD. Practical Pain Management, 21(4).
Cosio D & Lin E. (2020). Hypnosis: Tool for pain management. Practical Pain Management, 15(4). https://www.practicalpainmanagement.com
Crozer Health. (2022). PQRST pain assessment method. https://www.crozerhealth.org
Curfman G. (2019). FDA strengthens warning that NSAIDs increase heart attack and stroke risk. Harvard Health Publishing. https://www.health.harvard.edu
Dafny N. (2020). Chapter 8: Pain modulation and mechanisms. In Neuroscience Online. University of Texas. https://nba.uth.tmc.edu
Dance A. (2019). Why the sexes don’t feel pain in the same way. Nature, 567, 448–50. https://doi.org/10.1038/d41586-019-00895-3
Department of Health, Western Australia (DHWA). (2021). Yoga and pain. https://painhealth.csse.uwa.edu
DeWeerdt S. (2019). Tracing the U.S. opioid crisis to its roots. Nature. https://www.nature.com
Dhaliwal A & Gupta M. (2021). Physiology, opioid receptor. StatPearls [Internet].
Doctors.net/uk. (n.d.). Table 1: Pros and cons of different routes of drug administration. https://www.doctors.net.uk
Dowell D, Ragan KR, Jones CM, Baldwin GT, & Chou R. (2022). CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep, 71(RR-3), 1–95. http://dx.doi.org/10.15585/mmwr.rr7103a1
Edward R. (2021). Chap. 2: Assessment and measurement of pain and pain treatment. In Acute pain management: scientific evidence (5th ed). https://www.anzca.edu
Eisenberg G. (2021). More insight on the role of personality traits and sensitivity to experimental pain. Journ. of Pain Research, 14, 1837–44. https://doi.org/10.2147/JPR.S309729
Girgis L. (2021). Is your patient a drug seeker? 13 red flags to watch for. Medical Economics. https://www.medicaleconomics.com
Glasofer D. (2021). What is acceptance and commitment therapy (ACT)? Verywellmind. https://www.verywellmind.com
Gray R & Whalley B. (2020). The proposed mechanism of action of CBD in epilepsy. Epileptic disorders, 22(S1), S10–15. https://onlinelibrary.wiley.com
Hauer J & Jones B. (2021). Evaluation and management of pain in children. UpToDate. https://www.uptodate.com
Hoenig H & Cary M. (2021). Overview of geriatric rehabilitation: Program components and settings for rehabilitation. UpToDate. https://www.uptodate.com
International Association for the Study of Pain (IASP). (2021a). Access to pain management: Declaration of Montreal. http://www.iasp-pain.org
International Association for the Study of Pain (IASP). (2021b). IASP curriculum outline on pain for occupational therapy. https://www.iasp-pain.org
International Association for the Study of Pain (IASP). (2021c). IASP curriculum outline on pain for physical therapy. https://www.iasp-pain.org
Jackson D. (2020). What nurses should know about opioids. Ottawa University. https://www.ottawa.edu
Jacques E. (2021). Idiopathic pain is well known even if its cause isn’t. Verywellhealth. https://www.verywellhealth.com
Jakucs C. (2021). Drug-seeking behavior is a challenge for any ED. Nurse.com
Jalali M, Botticelli M, Hwang R, Koh H, & McHugh K. (2020). The opioid crisis: A contextual, social-ecological framework. Health Res Policy Sys, 18, 87. https://doi.org/10.1186/s12961-020-00596-8
Jamison R & Craig K. (2022). Chapter 11: Psychological assessment of persons with chronic pain. In Lyn M, Craig K, & Peng P (eds.), Clinical pain management: a practical guide (2nd ed.). John Wiley & Sons.
Johns Hopkins Medicine. (2022). Palliative care methods for controlling pain. https://www.hopkinsmedicine.org
Johnson TR. (2017). Managing the drug-seeking patient. Curi. https://curi.com
Kahn Academy. (2019). Somatosensory homunculus. https://www.khanacademy.org
Keane K. (2021). What is distraction therapy (for pain)? Arthritis New South Wales. https://www.arthritisnsw.org
Kishner S. (2018). Pain assessment. Medscape. https://emedicine.medscape.com
Kim J & DeJesus O. (2022). Medication routes of administration. StatPearls.
KnowledgeDose. (2020). Routes of drug administration. https://www.knowledgedose.com
Lee E. (2022). How to identify pain in care settings. CPD Online College. https://cpdonline.co.uk/knowledge-base/care/identify-pain-care-settings/
Madore A. (2020). Therapeutic massage for pain relief. Brigham Health Hub. https://brighamhealthhub.org
Mahajan G. (2021). Urine drug testing for patients with chronic pain. UpToDate. https://www.uptodate.com
Mandall A. (2019). Opioid side effects. News-Medical.net
Marks JL. (2022). Qigong and tai chi to help chronic pain. Everyday Health. https://www.everydayhealth.com
Martin D, et al. (2019). Chapter 5: Pain assessment and management in children. In MJ Hockenberry, D Wilson, & CC Rodgers (Eds.), Wong’s nursing care of infants and children (11th ed.). Elsevier.
Maryland Board of Nursing (MD BON). (n.d.). Pain management: Nursing role/core competency. A guide for nurses. https://mbon.maryland.gov
Mayo Clinic. (2022). Acupuncture. https://www.mayoclinic.org
Mayo Clinic. (2021). Tests and procedures. https://www.mayoclinic.org
Medicine LibreTexts. (2020). Components of a reflex arc. https://med.libretexts.org
Meldrum M. (2021). Pain. Britannica. https://www.britannica.com
Mills S, Nicolson K, & Smith B. (2019). Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J. Anaesth, 123(2), E273–83.
Mlost J, Bryk M, & Starowicz K. (2020). Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Int J. Mol Sci, 21(22), 8870. doi:10.3390/ijms21228870
Murphy D. (2020). Ultrasound in physical therapy: Does it work? PTProgress. https://www.ptprogress.com
National Center for Complementary and Integrative Health (NCCIH). (2022). Pain. https://nccih.nih.gov
National Center for Complementary and Integrative Health (NCCIH). (2021). Relaxation techniques: What you need to know. https://www.nccih.nih.gov
National Institute on Drug Abuse (NIDA). (2022a). What is the scope of prescription drug misuse in the United States? https://nida.nih.gov
National Institute on Drug Abuse (NIDA). (2022b). Screening and assessment tools chart. https://nida.nih.gov
National Institute on Drug Abuse (NIDA). (2020a). Cannabis (marijuana) research report. https://nida.nih.gov
National Institute on Drug Abuse (NIDA). (2020b). Opioid crisis and pain management. https://www.drugabuse.gov
National Library of Medicine (NLM). (2022a). Acetaminophen. PubChem. https://pubchem.ncbi.nlm.nih.gov
National Library of Medicine (NLM). (2022b). Iontophoresis with dexamethasone and physical therapy to treat apophysitis of the knee in pediatrics. ClinicalTrials.gov
National Library of Medicine (NLM). (2021). Pain and your emotions. MedlinePlus. https://medlineplus.gov
National Survey on Drug Use and Health (NSDUH). (2020). Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. https://nsduhweb.rti.org
Nnanna O. (2021). Blood tests to identify medical causes of neuropathy. Neuropathy Commons. https://neuropathycommons.org
NORML. (2022). Oregon medical marijuana law. https://norml.org
O’Connor M. (2020). New PET/MRI method spots chronic pain points, alters more than half of management plans. HealthImaging. https://www.healthimaging.com
Oregon Alcohol and Drug Policy Commission (OR ADPC). (2022). Opiates or opioids—what’s the difference? Oregon.gov
Oregon Secretary of State (OSS). (2018). Audit highlights. https://sos.oregon.gov
O’Sullivan S, Schmitz T, & Fulk G. (2019). Physical rehabilitation (7th ed.). F. A. Davis.
Padgett A. (2019). Do it for your mind-if not for your body: The psychological effects of chronic pain. Augusta Pain Center. https://augustapaincenter.com
Physiopedia. (2022b). Cryotherapy. https://www.physio-pedia.com
Physiopedia. (2022c). Behavioural approaches to pain management. https://www.physio-pedia.com
Physiopedia. (2022d). Mirror therapy. https://www.physio-pedia.com
Physiopedia. (2021). Therapeutic modalities. https://www.physio-pedia.com
Portenoy R, Ahmed E, & Keilson Y. (2022). Cancer pain management: Role of adjuvant analgesics (coanalgesics). UpToDate. https://www.uptodate.com
Portenoy & Copenhaver. (2021). Cancer pain management: Interventional therapies. UpToDate. https://www.uptodate.com
Portenoy R & Dhingra L. (2020). Assessment of cancer pain. UpToDate. https://www.uptodate.com
Portenoy R, Mehta Z, & Ahmed E. (2022a). Prevention and management of side effects in patients receiving opioids for chronic pain. UpToDate. https://www.uptodate.com
Portenoy R, Mehta Z, & Ahmed E. (2022b). Cancer pain management with opioids: Optimizing analgesia. UpToDate. https://www.uptodate.com
Practical Pain Management (PPM). (2019). Manipulation and massage for pain management. https://patient.practicalpainmanagement.com
Project CBD. (2022). How CBD works. https://www.projectcbd.org
Rababa M, Sabbaj A, & Havajneh A. (2021). Nurses’ perceived barriers to and facilitators of pain assessment and management in critical care patients: A systematic review. Journal of Pain Research, 14, 3475–91.
Ratka A. (2020). Pharmacogenomics in the context of pain management. St. John Fisher College. https://www.urmc.rochester.edu
Reeves L, Sako M, Malloy J, Goldstein E, & Bennett K. (2022). Role of occupational therapy in comprehensive integrative pain management. AOTA. https://www.aota.org
Rosenquist R. (2022). Revised screener and opioid assessment for patients with pain (SOAPP-R). UpToDate. https://www.uptodate.com
Schiller E, Goyal A, & Mechanic O. (2022). Opioid overdose. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov
Schreiber J & Culpepper L. (2022). Suicidal ideation and behavior in adults. UpToDate. https://www.uptodate.com
Seidel A, Chang L, & Greenberg A. (2021). Therapeutic modalities. PM&R Knowledge NOW. https://now.aapmr.org
Sevarino K. (2022). Opioid withdrawal in adults: Clinical manifestations, course, assessment, and diagnosis. UpToDate. https://www.uptodate.com
Slater H & Davies S. (2021). Pain types. painHEALTH. https://painhealth.csse.uwa.edu
Solomon D. (2022). Patient education: Nonsteroidal anti-inflammatory drugs (NSAIDs). UpToDate. https://www.uptodate.com
Stanford Health Care. (2021). Management of pain without medications. https://stanfordhealthcare.org
Stonebraker C. (2022). Is marijuana legal, illegal or decriminalized near you? And what does that mean? GoodRx Health. https://www.goodrx.com
Substance Abuse and Mental Health Services Administration (SAMHSA). (2021). Managing chronic pain in adults with or in recovery from substance use disorders. Advisory. Publication No. PEP20-02-01-022.
Tauben D & Stacey B. (2022). Evaluation of chronic non-cancer pain in adults. UpToDate. https://www.uptodate.com
The Joint Commission (TJC). (2021). Pain assessment and management—understanding the requirements. https://www.jointcommission.org
Tinnirello A, Mazzoleni S, & Santi C. (2021). Chronic pain in the elderly: Mechanisms and distinctive features. Biomolecules, 11, 1256. https://doi.org/ 10.3390/biom11081256
Toney-Butler T. (2019). Nursing admission assessment and examination. StatPearls [Internet]. https://www.statpearls.com
U.S. Drug Enforcement Administration (U.S. DEA). (2019). Drug addiction in health care professionals. https://www.deadiversion.usdoj.gov
U.S. Food and Drug Administration (U.S. FDA). (2022). FDA approved drugs. https://www.accessdata.fda.gov
U.S. Food and Drug Administration (U.S. FDA). (2021). Abuse-deterrent opioid analgesics. https://www.fda.gov
U.S. Food and Drug Administration (U.S. FDA). (2020). Drug disposal: FDA’s flush list for certain medicines. https://www.fda.gov
University of Florida Health (UFHealth). (2022). Pain assessment scales/tools. https://pami.emergency.med.jax.ufl.edu
University of Georgia (UGA). (2020). Survey of marijuana law in the United States: History of marijuana regulation in the United States. https://libguides.law.uga.edu
University of Rochester Medical Center (URMC). (2022). TENS therapy. https://www.urmc.rochester.edu
Victoria Department of Health. (2021). Identifying pain. https://www.health.vic.gov
VirtualTimes. (2021). How does our brain respond to virtual reality? https://virtualtimes-h2020.eu/how-does-our-brain-respond-to-virtual-reality/
Washington State Health Care Authority (WSHCA). (2021). Analgesics: Opioid agonists. https://www.hca.wa.gov
Watson J. (2022). Neuropathic pain. Merck Manuals. https://www.merckmanuals.com
Watson S. (2020). Overview of biofeedback. WebMD. https://www.webmd.com
Weber CG. (2022). Clinical pain management (the clinical medicine series, book 22) [Kindle version]. Clinical Medicine Consult. http://www.amazon.com
West Virginia Board of Pharmacy Prescription Monitoring Program (WVPMP). (2019). Dispensers/dispensing prescribers. https://www.nascsa.org
Wheeler T. (2021). How do doctors find the cause of pain? WebMD. https://www.webmd.com
Williams D. (2021). What are the benefits of pulse PEMF therapy? Interventional Orthopedics of Atlanta. https://ioaregenerative.com
Winchester Hospital. (2022). Moving meditation: The art of tai chi. https://www.winchesterhospital.org
World Health Organization (WHO). (2022a). Management of substance abuse. https://www.who.int






Customer Rating
4.9 / 645 ratings