Lung Cancer
Online Continuing Education Course

Course Description
Lung cancer is the third most common cancer in the United States and has the highest mortality rate. This CEU course provides information on lung cancer, including non-small cell lung cancer, adenocarcinoma, and mesotheliomas. Learn about signs, treatments, and therapies per stage, as well as recurrence.
"Current, evidence-based instruction. Thank you." - Mary, RN in Texas
"Excellent course. I will take more courses from Wild Iris." - Sue, RN in Illiniois
"Great course. I appreciate the opportunity to print the course content to read through and comprehend the information." - Mike, RN in Ohio
"The ability to complete this course and take the test at my own pace was extremely helpful due to a busy schedule." - Rosemary, RN in Florida
LUNG CANCER
Copyright © 2022 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this course, you will have increased your understanding of the causes of and the current treatments for lung cancer. Specific learning objectives to address potential knowledge gaps include:
- Discuss the epidemiology of lung cancer.
- Describe the pathophysiology of lung cancer.
- Recognize the risk factors and etiology of lung cancer.
- Explain the clinical manifestations and assessment of the patient with lung cancer.
- Comprehend the types and staging of primary lung cancer.
- Summarize the primary lung cancer treatment modalities.
- Discuss the elements of rehabilitation therapy.
- Identify the complications that can result from lung cancer.
- Explain common palliative care treatments.
- Describe survivorship and follow-up care for patients and their families.
TABLE OF CONTENTS
- Introduction
- Epidemiology
- Pathophysiology of the Lung
- Etiology and Risk Factors
- Patient Assessment and Diagnosis
- Types and Staging of Lung Cancer
- Lung Cancer Treatment Modalities
- Rehabilitation Therapy
- Complications of Lung Cancer
- Palliative Care
- Survivorship and Follow-Up Care
- Conclusion
- Resources
- References
INTRODUCTION
Lung cancer is a disease in which some cells of the respiratory system exhibit abnormal growth. These abnormal cells may then metastasize to the lymph system or to other organs such as the brain, liver, bones, adrenal glands, or the other lung.
Lung cancer includes two main types: non-small cell lung cancer (NSCLC) and small cell lung (SCLC). Non-small cell carcinoma is much more common, accounting for 84% of lung cancers, with small cell lung cancer accounting for 13% (ACS, 2020a; CDC, 2019a). In this course, all cancers that originate from the respiratory tract will be referred to as lung cancer.
Advances in diagnostic testing and screening have resulted in earlier recognition and, therefore, earlier treatment and cures of all cancers, including lung cancer. The most recent treatment modalities have improved outcomes of patients with cancer in general, but the occurrence and mortality of lung cancer persists.
EPIDEMIOLOGY
Incidence and Mortality
The third most common cancer in the United States is lung cancer, according to recent statistics from the Centers for Disease Control and Prevention (CDC). The most common form of cancer is skin cancer. Breast cancer (among women) and prostate cancer (among men) are the next most common cancers in the United States. Lung cancer has the highest mortality among cancers in the United States, regardless of gender (CDC, 2021c). The greatest risk of lung cancer is from smoking, though lung cancer can occur in people who have never smoked.
Lung cancer incidence has been declining since the mid-1980s in men but only since the mid-2000s in women because of gender differences in historical patterns of smoking uptake and cessation. Since the mid-2000s, incidence has decreased steadily by about 2% per year overall but at a faster pace in men than in women. Lung cancer mortality has declined by 54% since 1990 in men and by 30% since 2002 in women due to reductions in smoking, with the pace accelerating in recent years; from 2014 to 2018, the rate decreased by more than 5% per year in men and 4% per year in women (ACS, 2021a).
| Sex | Estimated New Cases | Estimated New Deaths |
|---|---|---|
| (ACS, 2021a) | ||
| Male | 119,100 | 69,410 |
| Female | 116,660 | 62,470 |
| Both sexes | 235,760 | 131,880 |
Disparities by Sex
Studies of more than 450,000 patients over a period of eight years have consistently shown that men experience lung cancers at a higher rate than women. Historically, this was due to the fact that men smoked much more than women. This has progressively become less so, to the point that the levels of smoking are equal between the two sexes. Regardless of the various confounding variables (lung cancer types, smoking histories, and socioeconomic backgrounds allowing access to care) men were consistently found to have a higher incidence of lung cancer than women (Tolwin et al., 2020).
Cultural and Ethnic Disparities
Recent statistics of rates of lung cancers among various races and ethnicities show the incidence between Black people and White people to be almost equal. The next most common occurrence is among American Indian/Alaskan Natives, followed by Asian/Pacific Islanders, and finally, Hispanics, at less than half the prevalence of Whites and Blacks (see table below).
| Race/Ethnicity | Rate per 100,000 |
|---|---|
| (ACS, 2021a) | |
| Non-Hispanic White | 62.6 |
| Non-Hispanic Black | 60.9 |
| American Indian/Alaskan Native | 52.7 |
| Asian/Pacific Islander | 34.4 |
| Hispanic/Latinx | 29.7 |
In a study of approximately 1,200,000 subjects, the measurement of metastatic presentation upon the diagnosis of non-small cell lung cancer (NSCLC) was remarkably similar across ethnic strata. The results showed that 47% of Black study subjects, 49.66% of Hispanic subjects, and 42.33% of White subjects originally presented to a physician or advanced practice provider with symptomatic complaints that were caused by stage IV disease with metastasis to another organ system (Aghdam et al., 2020).
Prevalence by Age
Lung cancer is predominantly a disease of the elderly. Incidence of new cases and overall prevalence jump dramatically in adults over the age of 50, with the highest rate of new cancers among those 80–84 years (CDC, 2021c). Generally, women develop lung cancer at a younger age than men. On the average, women with lung cancer live one year longer than men (Harding et al., 2020).
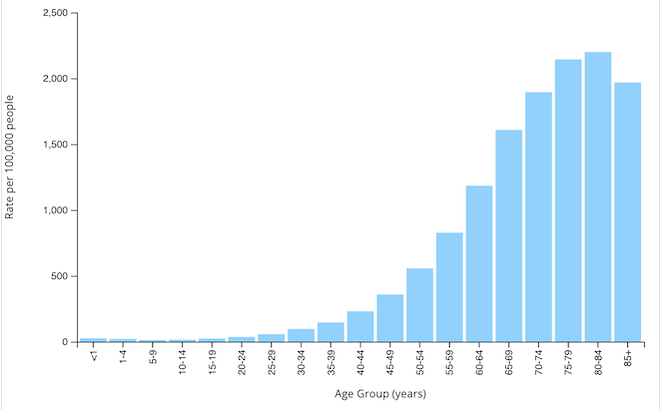
Rate of new lung and bronchus cancers by age group, U.S., 2019. (Source: CDC, 2021c).
LUNG CANCER AND LIFE EXPECTANCY
Due to the late stages of the cancer upon diagnosis, lung cancer has a high mortality and a low cure rate. These have improved somewhat in recent years because of less invasive surgical treatments such as video-assisted or robot-assisted thoracoscopy (VAT or RAT), more direct radiation therapy, and more effective systemic therapies (e.g., chemotherapy, immunotherapy, and targeted therapy) (Harding et al., 2020).
PATHOPHYSIOLOGY OF THE LUNG
Normal Lungs
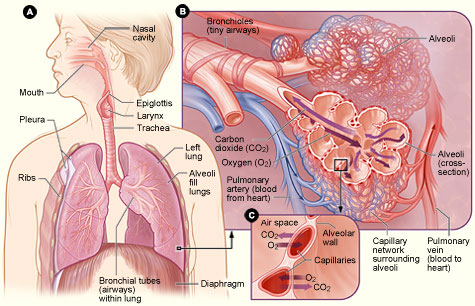
Figure A: Locations of the respiratory structures in the body.
Figure B: Enlarged image of airways, alveoli, and their capillaries in the lungs.
Figure C: Location of gas exchange between the capillaries and alveoli.
(Source: National Institutes of Health.)
LUNG STRUCTURE
The lungs are a pair of cone-shaped organs in the thoracic cavity. Each lung has three surfaces: anterior, posterior, and inferior. The right lung is larger and weighs more than the left lung, and it has three lobes. The left lung has two lobes and a space for the heart, called a cardiac notch (Physiopedia, 2020).
Each type of lung tissue is differentiated from the others. Mature, differentiated cells are distinguished from other types by a unique appearance as well as function.
Pleura
Both lungs are surrounded by a serous pleural sac comprised of two continuous membranes. There are four components of the parietal pleura: 1) the costal pleura line the thoracic wall; 2) the mediastinal pleura line the lateral mediastinum; 3) the diaphragmatic pleura line the superior diaphragm and both sides of the mediastinum; and 4) the cervical pleura extends to the neck and covers the apices of the lungs (Physiopedia, 2020).
Pleural Cavity
The pleural cavity is a potential space between the parietal and visceral layers of the pleura. Serous fluid in the pleural cavity lubricates the two surfaces of the pleura so that they can move easily over each other during respirations. Surface tension in the pleural cavity causes the lungs to stick to the thoracic wall and leads to a close proximity of the lung surfaces with the chest all, which supports more inflation of the alveoli during inspiration (Physiopedia, 2020).
Lung Fissures
Each lung is divided by fissures (connective tissue walls) into separate lobes. Both lungs have oblique fissures dividing the lobes, and the right lung has an additional, transverse fissure that forms the third lobe (Physiopedia, 2020).
Bronchial Tree
At the top of the bronchial tree is the trachea. The trachea divides into the right and left primary bronchus or main bronchi. Each bronchus enters the lung at a notch called the hilum. The right main bronchus is less angled and has a larger diameter than the left and exits the trachea at a higher level than the left main bronchus. Anything foreign that enters the bronchial tree will tend to enter into the right main bronchus rather than the left, primarily due to size and position. The main bronchi further divide into secondary and then tertiary bronchi. The tertiary bronchi supply the bronchopulmonary segments with air (Physiopedia, 2020).
Bronchopulmonary Segments
The bronchopulmonary segments are the largest divisions of a lobe. The tertiary bronchi further subdivide into smaller bronchioles. Each of these continue to subdivide into 50–80 even smaller bronchioles. Eventually, the terminal bronchioles form the approximately 300 million alveolar ducts and sacs, where the exchange of oxygen and carbon dioxide takes place during respirations (Physiopedia, 2020).
Bronchopulmonary Segment Histology
The trachea is surrounded by cartilage rings, smooth muscles, and elastic fibers. Goblet cells for mucus production are lined with ciliated epithelia shaped like columns, which aid in secretion removal. The smaller bronchioles have elastic tissues and smooth muscle fibers. The alveoli are fashioned of thin squamous epithelial cells. The gas exchange of oxygen and carbon dioxide takes place between these cells (Physiopedia, 2020).
LUNG FUNCTION
Respirations allow the exchange of oxygen (O2) and carbon dioxide (CO2). Oxygen is inhaled from the atmosphere to the alveoli, where the gas exchange takes place. In the alveolar capillary membranes, venous blood becomes oxygenated arterial blood that returns to the body. The process of oxygenation takes place via ventilation, perfusion, and diffusion.
Ventilation is the movement of gases in and out of the lungs. Perfusion is the cardiovascular system pumping oxygenated blood to the tissues and deoxygenated blood back to the lungs, where it will be reoxygenated. Diffusion is the exchange of respiratory gases in the alveolar capillary membranes. Surfactant is a chemical that serves to keep the alveolar sacs open so that they may fill with inhaled air.
The accessory muscles of ventilation (the scalene, sternocleidomastoid, pectoralis major, trapezius, and external intercostal muscles) can increase the lungs’ volumes. Excessive use of these muscles causes fatigue. Compliance is the ability of the lungs to expand or distend as a result of intra-alveolar pressure. Airway resistance is the increase in pressure as the diameter of the airway becomes progressively smaller along the passage of air from the mouth or nose to the alveoli. Tumors in the airway will also increase airway resistance. The increased use of accessory muscles, decreased lung compliance, and increased airway resistance result in the increased work of breathing, causing increased energy expenditure and oxygen consumption (Potter et al., 2019).
Lungs with Lung Cancer
The majority of primary lung tumors originate in mutated epithelial tissue that was previously normal tissue. Mutated tissue differs in behavior and appearance from normal tissue. In a normal cell, it is the DNA that controls the growth and behavior of new cells. Cancer can cause the DNA to create cells that are abnormal in appearance and to reproduce more rapidly (NCCN, 2019).
There are several different types of lung cancers, categorized based on the cellular and molecular characteristics of the cancer cells present (histopathological class). It is vitally important to know the histopathology of the lung cancer to correctly diagnose and treat the cancer (Siddiqui et al., 2021). (See also “Types and Staging of Lung Cancer” later in this course.)
Carcinogenic factors cause the mutations, depending on genetic factors. The slow tumor development is fostered by epidermal growth factors. It takes 8–10 years for a lung tumor to grow large enough to be detected by X-ray. Lung cancer tumors usually arise in the segmental bronchi or the upper lobes (Harding et al., 2020).
ETIOLOGY AND RISK FACTORS
Research has shown that specific risk factors increase a person’s chance of developing lung cancer. Despite the risks involved, it is not always possible to predict precisely why one person develops lung cancer and someone with similar risk factors does not. For example, advanced age and male gender contribute to a higher occurrence of lung cancer, but other risk factors may turn out as more causative.
Smoking
Smoking is by far the number one risk factor for lung cancer. This includes cigarettes, cigars, and vaping. Up to 80%–90% of all cases of lung cancer are caused by current or former smoking. The remaining 10%–20% of cases occur in people who have never smoked (“never smokers”) (ALA, 2020a; CDC, 2021b).
Tobacco contains approximately 7,000 chemicals, many of which are poisons. Seventy of these chemicals are tied to cancers in humans and animals. Eighty to ninety percent of people who contract lung cancer have a history of smoking. A person who has smoked a few cigarettes or smoked for a brief period of time is at higher risk for lung cancer than individuals who have never smoked. Smokers are 15 to 30 times more likely to develop lung cancer than nonsmokers (CDC, 2019a).
PACK-YEARS
A person’s smoking intensity is measured in pack-years. One “pack-year” is defined as smoking approximately one pack (20 cigarettes) per day for one year. Smoking half pack a day for one year is equivalent to 1/2 pack-years, and smoking two packs a day for 10 years is equivalent to 20 pack-years. The higher a person’s number of pack-years, the more likely it is that they will develop conditions such as lung cancer, emphysema, bronchitis, or heart diseases.
CASE
Earlene is a 72-year-old smoker who presented at the clinic with a complaint of almost continuous coughing and shortness of breath (SOB) for 3–4 weeks with no production of sputum. When taking her medical history, the nurse practitioner, Caitlyn, discovers that Earlene has been smoking since age 12, with no significant break in her smoking. When questioned, Erlene tells Caitlyn that she has smoked an average of 10–15 cigarettes a day (or 1/2 to 3/4 pack) for all of those 60 years. The nurse explains the term pack-year to Earlene and tells her that she has a smoking history of 30 to 45 pack-years, which is a significant finding. Caitlyn refers Earlene for follow-up and diagnostic testing.
Exposure to second-hand smoke (the smoke exhaled by another) is a significant risk factor for lung cancer. Exposure to secondhand smoke causes more than 7,300 deaths per year in nonsmokers. Repeated exposure is a chronic irritant that can cause diseases or infections. In the United States 1 out of 4 people, including 14 million children, who don’t smoke are exposed to second-hand smoke. It also contributes to heart disease and stroke in adults (CDC, 2021a).
Tobacco smoke in the air indoors settles on the floor and other surfaces or is released into the air in a process called off-gassing. Residual nicotine and other toxic chemicals build up in the air or on clothing, rugs, furniture, bedding, dust, and vehicle surfaces and mixes with other pollutants, causing a carcinogenic composite. This residue, referred to as third-hand smoke, doesn’t dissipate over time and must be aggressively cleaned or laundered. Children are at risk for the development of lung cancer from third-hand smoke, as they tend to put things in their mouths and touch affected surfaces. Their smaller lungs are especially sensitive to off-gassing (Hayes, 2017).
Vaping, also referred to as electronic or e-cigarettes, has become a common replacement or alternative to smoking conventional cigarettes or marijuana. Electronic cigarettes have been globally popular for the past 15 years. Originally, they were thought to be a reasonable way to reduce or gradually wean oneself from smoking nicotine-based cigarettes. It has since been discovered that e-cigarettes can be equally addicting as regular cigarettes and cause lung injuries, including cancer. They contain propylene glycol, a known lung irritant, and sufficient levels of nicotine to be addictive and carcinogenic (CASAA, 2020).
LUNG CANCER SCREENING
The use of computerized tomography (CT) scanning for lung screening has provided the ability to diagnose very small tumors that can be excised by a segmentectomy. This allows removal of the smallest amount of lung tissue possible and preserves the most pulmonary function. Robotic segmentectomy is more complex than robotic lobectomy depending on the ability to access the tumor with the robotic arms.
The U.S. Preventive Services Task Force recommends yearly lung cancer screening with low-dose CT for people who:
- Have a 20 pack-year or more smoking history, and
- Smoke now or have quit within the past 15 years, and
- Are between 50 and 80 years old
(CDC, 2021d)
Environmental Pollutants
The inhalation of various pollutants, both particles and gases, can contaminate the lungs. Large particles are expelled by coughing or sneezing, but tiny particles remain in the lungs, and even brief exposure to these particles in large enough amounts can cause the development of lung cancer (ALA, 2020b).
- Radon gas, a naturally occurring, odorless, tasteless radioactive gas that can become trapped inside of buildings, causes approximately 20,000 cases of lung cancer every year (ALA, 2020a; CDC, 2019a).
- Small mineral fibers such asbestos, found in the workplace and in older residences, are known to cause lung cancer, mesothelioma, and other pulmonary disorders, and can be mediated by the use of personal protection equipment such as masks and gloves, reduction of exposure time, and the education of employees regarding safe practices (Vuong, 2020).
- Air pollution can cause an increase in the burden of disease from stroke, heart disease, lung cancer, and asthma unless measures are taken in the areas of transport, urban planning, power generation, and industry that reduce air pollution (WHO, 2018).
Occupational Exposures
There are many toxic substances that can lead to lung cancer with prolonged exposure in an occupational environment. Preventative processes and personal protective equipment (PPE) have been developed and introduced to protect workers who experience contact with these substances from developing lung cancer.
- Polycyclic aromatic hydrocarbons, including industrial coke, silicon, carbon products, foundry and combustion processes, lubrication oils and engine exhaust emissions, and bitumen in larger exposure amounts, are a health hazard to millions of workers globally (Petit et al., 2019).
- Coal dust and other coal mining–related toxins have been associated with an elevated risk of lung cancers and pneumoconiosis (black lung) when inhaled (Green & McGinley, 2019).
- Nickel and chromium in welding fumes have been linked to the development of lung cancer in welders (Pesch et al., 2019).
- Uranium leads to a high incidence of lung cancer, including from inhaling radon degradation products (RDP), uranium dust, and external gamma rays during uranium mining, and due to exposure when it is processed, refined, or milled for use in nuclear energy (Zablotska et al., 2018).
- Arsenic is associated with cancers of the lung, bladder, skin, kidneys, nasal passages, liver, and prostate, which has led the Environmental Protection Agency (EPA) to reduce the maximum contaminant level allowed in public water systems (EPA, 2020).
Family History and Genetics
The risk of having lung cancer is higher if one’s parents, siblings, or children have lung cancer. This may be due to an unexplained familial tendency. There may also be common exposure to carcinogens when family members live or work together (CDC, 2019a).
Ten percent of all cancers have a link to hereditary or genetic traits. The most common genetic cause of lung cancer is epidermal growth factor receptor (EGFR) gene mutations. EGFR accounts for 10% of all small cell cancers, and it is believed that 50% of all lung cancers not caused by smoking are related to EGFR. Other genetic mutations that have recently been determined to cause lung cancer are ALK, ROS1, and BRAF. These findings have driven the direction of pharmacological research for the treatment of lung cancer (Howley, 2019).
When lung cancer is caused by genetic changes, the cancer risk follows an autosomal dominant pattern, which means one copy of the altered gene in each cell will increase a patient’s chance of getting the disease. Patients who inherit this altered gene have an increased risk of cancer. Not all people who inherit mutations in these genes will develop lung cancer, but a small percentage do (NIH, 2020b).
Patients with cancers linked to hereditary or genetic trains are at higher risk for developing multiple cancers. These patients are also more likely to develop cancer at a younger age and to be diagnosed at a more advanced stage (CDC, 2019b).
EPIDERMAL GROWTH FACTORS
The epidermal growth factor receptor (EGFR) protein contributes to cell-signaling pathways that govern a cell’s ability to replicate and continue to live. Mutations in the EGFR gene cause the proteins to be made in larger-than-normal amounts on some types of cancer cells. This causes the cancer cells to replicate more rapidly. Drugs that interfere with epidermal growth factor receptor proteins, such as osimertinib (Tagrisso), are being used in the treatment of some types of cancers to prevent the rapid proliferation of the abnormal cancer cells (NCI, 2020a).
CASE
Dwight is a 47-year-old construction worker who has just been diagnosed with a small NSCLC tumor in his left lower lobe. He has never smoked. During his intake history and physical, it is discovered that his father died at age 38 of lung cancer, as did his paternal grandfather. Dwight told his physician that he did not know how long or how much each family member smoked but that they smoked “all their lives.” When questioned about possible exposure to carcinogenic substances on the job, such as asbestos or radon, Dwight stated that he was not aware of any, but that “it’s certainly possible.”
As part of Dwight’s lung biopsy to definitively diagnose his cancer, the surgeon sends some of the sample to test for EGFR DNA mutation, given the multigenerational family history of lung cancer and Dwight’s own lack of smoking. The test returns as positive. Dwight’s surgeon explains the combination of treatments possible, such as osimertinib and surgery, to treat his NSCLC and connects Dwight with the nurse, who can help him make a follow-up appointment to discuss treatment options further.
HORMONE REPLACEMENT THERAPY AND LUNG CANCER
The research on the possibility of whether there is a relationship between women taking hormone replacement therapy (HRT) after menopause and the occurrence of lung cancer has produced divergent findings. A 2019 meta-analysis of research into this topic discovered that previous findings were inconclusive and that there was not a definitive correlation or causative effect between the use of HRT and lung cancers (Jin & Lang, 2019).
Other Medical Conditions
Several other medical conditions and their treatment modalities may be risk factors for the development of lung cancer. Lung cancer is also often detected during diagnostic testing for these conditions. Such coexisting conditions may precede the appearance of malignant tumors or may be discovered during the testing for the cancerous symptoms.
EMPHYSEMA / CHRONIC BRONCHITIS
With emphysema, the alveoli (air sacs) in the lungs become damaged and their inner walls rupture, causing a decrease in lung surface area, leading to hypoxia. This compromises exhalation, causing air to become trapped in the chest (Mayo Clinic, 2020a). The severe airway obstruction experienced in emphysema makes a person two to three times as likely to develop lung cancer. Certain phenotypes of emphysema (e.g., centrilobular) are associated with a greater incidence of lung cancer. Parastatal emphysema has proved to be less likely to coexist with lung cancer (Gonzales et al., 2019).
Chronic bronchitis is the condition of limited or obstructed airflow due to chronic inflammation. It presents as excessive coughing with sputum production for a period of three months or more in at least two consecutive years.
BRONCHIECTASIS
Bronchiectasis is a pulmonary condition that causes the airways to become inflamed and scarred in the presence of excessive sputum. The most common symptoms are dyspnea, productive cough, hemoptysis, wheezing, chest pain, clubbing, weight loss, fatigue, and pulmonary infection. Bronchiectasis is commonly associated with smoking, the most common cause of lung cancer. Bronchiectasis may be present in 10%–15% of patients with lung cancer (Sanchez-Carpintero Abad et al., 2020).
SOLID ORGAN TRANSPLANT
In a study of 463 patients who received lung transplantation over a period of 25 years, the relationship between lung transplantation and lung cancer was explored. There were 19 patients in the study who were found to have lung cancer. The majority of the cancers were found in the explanted lung (lung to be removed) or the lung not needing transplantation. Only 3 of the 19 positive cases were found to have malignant tumors in the transplanted lung.
Only 2.37% of the patients in the study were found to have lung cancer in transplanted, explanted, or unaffected lungs. They also determined that the life expectancy of the patients in the study without lung cancer was an average of 8.1 years. Those patients with lung cancer had an average life expectancy of 5–6 years (Chatron et al., 2019).
Following lung transplantation, cancer is the second most common cause of death (17.3%) in the 5–10 year postoperative period. Patients who have had solid organ transplantation (SOT) are placed immediately on large-dose immunosuppressive therapy to prevent organ rejection. Lung transplant recipients are given even more immunosuppressants than other organ transplant recipients. This reduces the effectiveness of antitumor immune surveillance, causing the increased percentage of cancer of the lung post lung transplantation (Shtraichman & Ahya, 2020).
TUBERCULOSIS
Tuberculosis is one chronic lung disease that complicates the course of lung cancer. It is likely that chronic inflammation in the lungs due to tuberculosis could cause clastogenic activity (or mutation or the breakdown of hormones) in the DNA of the bronchial epithelial tissue. Another possibility is lateral gene transfer; since Mycobacterium tuberculosis (MTb) is an intracellular organism, bacterial DNA could assimilate to bronchial epithelial tissue and cause a tumor to form neoplastic transformation (Molina-Romero et al., 2019).
LEUKEMIA / LYMPHOMA
Chemical air pollutants such as NO2 and CO and industrial substances such as styrene have been proven to cause both lung cancer and leukemia, sometimes both at the same time in the same patient. Occasionally, exposure to these types of chemicals may cause an increase the mortality of lung cancer and leukemia (Christensen et al., 2017; Dehghani et al., 2017).
It is not uncommon for patients to have a combination of lung cancer and lymphoma. One such condition is anaplastic lymphoma kinase (ALK)–rearranged advanced non-small cell lung cancer (Song et al., 2020).
The therapies used to treat leukemia and lymphoma, particularly among children, may have the late, secondary effect of causing lung cancer as an adult. A systematic review of the long-term effects of extended radiation therapy, particularly in the cases of Hodgkin lymphoma and childhood cancers, proved to cause an increased risk of primary cancers secondary to the side effects of the original cancer treatments. The increased risk occurred in the development of breast and lung cancers (Journy et al., 2019).
PATIENT ASSESSMENT AND DIAGNOSIS
A patient assessment begins with a medical history and a physical examination. Data is also collected on the patient’s past and current diagnoses. These steps provide a baseline against which any future health changes can be compared. In addition to the history and physical exam, assessment may involve laboratory testing and other diagnostics, including imaging, tumor biopsy, and pulmonary function.
Assessment involves a multidisciplinary team, including primary care providers, nurses, pharmacists, physical therapists (PT), occupational therapists (OT), respiratory therapists (RT), speech and language pathologists (SLP), medical social workers (MSW), and others.
Medical History
The medical history helps to determine the patient’s risk for disease and to diagnose any medical conditions, together with any subsequent laboratory or diagnostic test results. This is performed by a physician, advance practice nurse, resident, physician’s assistant (PA), or medical student (Harding et al., 2020).
The interview setting for taking a history must convey a climate of trust and respect to support effective and therapeutic communication. It is essential that the process be unrushed to ensure that the patient has enough time to remember details. Communication through facial expressions, body language, cultural considerations, and tone of voice are equally important to conduct a successful history-taking (Harding et al., 2020).
In the case of lung cancer, the medical history focuses on pulmonary symptoms, possible risk factors, and causative factors, such as smoking, exposure to other pathogens, and family history.
The type, amount, and duration of smoking is particularly important to note, since any exposure to smoke can be a precursor to lung cancer in the presence of other contributing factors. A history of exposure to smoke includes personal smoking, second-hand smoke, and third-hand smoke. (See also “Smoking” earlier in this course.)
If a lung cancer diagnosis is suspected, it is also important to ask about any possible exposure to carcinogens by inhalation, both environmental and occupational. This may include asbestos, radon, and industrial emissions. (See also “Environmental Exposure” and “Occupational Exposure” earlier in this course.) It is vital to question patients whose occupations may have included such exposures.
Physical Assessment
GENERAL APPEARANCE
When performing a physical examination, the first step is a general observation of the patient, including the patient’s voice, level of consciousness, ability to follow commands, ability to speak clearly and appropriately, and gross motor movements. For a patient with respiratory complaints, the observation of general appearance will include respiratory effort, use of accessory muscles of respiration, any audible respiratory sounds, respiratory rate, signs of fatigue, indications of cyanosis, and general strength.
HEART AND LUNG SOUNDS
Auscultation of the chest will reveal any abnormalities related to compromised airflow, blood flow, or cardiac rate. Electrical activity can be observed on a cardiac monitor. Fremitus (tactile vibrations produced by the voice and transmitted to the chest wall) can be blocked by excessive mucus, a collapsed lung, or a pulmonary lesion, making auscultation more difficult. Auscultation occurs anteriorly and posteriorly.
Neurological Assessment
The neurological assessment includes observing the mental status; determining whether there is normal or abnormal functional of the 12 cranial nerves; and testing motor and sensory function, cerebellar tasks, and reflexes. Medical history for a neurological patient would include rest/sleep patterns, medications, surgeries, neurological history, activity/exercise patterns, elimination, cognition, perception, and coping/stress patterns.
Clinical Manifestations
The clinical manifestations or symptoms of lung cancer can be discrete in early stages and may be mistakenly associated with some other illness. In later stages, lung cancer symptoms are much more pronounced. Understanding the clinical manifestations of lung cancer can help healthcare providers diagnose this serious health condition as soon as possible. Lung cancer is also often asymptomatic in early stages, highlighting the importance of screening when appropriate.
The most common presenting symptoms of all types of lung cancer are:
- A cough that doesn’t go away and gets worse over time
- Coughing up blood (hemoptysis)
- Shortness of breath or wheezing
- Chest pain
- Fatigue
- Loss of appetite or weight loss
- Hoarseness
- Swelling of the neck and face
(MedlinePlus, 2020a)
COUGH
A cough may be the earliest symptom that presents in lung cancer. It may be described as dry and tickling, or one may cough up mucus (called a productive cough). It can occur at any time of day and may have mechanical or environmental triggers. A chronic cough is defined as a cough that lasts for at least eight consecutive weeks, and many people with lung cancer say that they have a cough that “just won’t go away.” In one study of 223 patients with lung cancer, 57% had a cough at the time of diagnosis.
Coughing may be mistaken as resulting from smoking or other previous conditions such as bronchitis or allergies. This may mask the existence of cancer as the causative pathological process. A cough from lung cancer will be persistent, while one from smoking may be worse in the morning, as the lungs have filled with fluid during sleep.
Coughing has complex relationships with other symptoms, including breathlessness and fatigue, forming a symptom cluster (Molassiotis et al., 2017).
BLOOD-TINGED SPUTUM (HEMOPTYSIS)
Approximately 20% of all patients with lung cancer will experience some degree of hemoptysis. In fact, lung cancer accounts for 23% of all cases of hemoptysis.
There are many possible causes of hemoptysis in the presence of lung cancer, including neovascularization in the tumor, exfoliation of the tumor surface, necrosis of the tumor tissue, erosion of the airway into the surrounding vasculature, and bleeding after an airway procedure. The cancerous tissue or malignancy may be causing ruptured blood vessels that color the sputum, particularly in the presence of persistent coughing (Gershman et al., 2019).
SHORTNESS OF BREATH
Dyspnea (shortness of breath) is one of the most common symptoms of lung cancer. The type of symptoms that manifest may be related to the primary cancer type, the site or location within the lungs, and whether there is any metastasis. Dyspnea can be attributed to the malignant tumor compressing part of the airway or to hypoxia.
An early symptom of lung cancer, dyspnea may at first be attributed to overexertion, the aging process, or excess weight. Since there are so many medical diagnoses that include dyspnea as a symptom, the early occurrence of shortness of breath will not necessarily guide the healthcare provider to a diagnosis of lung cancer.
WHEEZING
Wheezing is the sound of air passing through an airway that is insufficiently sized for the volume of air. Compression on the airway or edema may contribute to the sound of wheezing. The sound resembles squeaking or has been described as a musical sound. This is caused by the rapid vibration of the bronchial walls. The sound is often loud enough to be auscultated without the use of a stethoscope.
CHEST PAIN
Chest pain is sometimes caused by pain from pleurisy or pleuritis, an inflammation caused by the tumor. The patient may describe the pain as sharp, dull, constant, intermittent, or becoming more acute with a deep breath. It may be confined to a specific area or felt throughout the chest. The discomfort may also be caused by enlarged lymph nodes or metastasis to the chest wall, the pleura, or the ribs (Healthline, 2021a). (See also “Pleural Involvement” below.)
FATIGUE
Fatigue is an enduring, subjective feeling of tiredness that interferes with daily functioning. Almost all patients with cancer experience fatigue. The most common causes of reversible fatigue in patients with cancer are depression, anemia, hypothyroidism, anxiety, insomnia, dehydration, and infection. It is essential for caregivers to recognize when the patient’s symptoms may be reversible in order to help initiate treatment.
The fatigue may be caused by the cancer itself causing pain or interfering with normal function. One of the most common causes of fatigue in patients with cancer is anemia. It may also be caused by the need for extra energy to promote healing.
NAUSEA / VOMITING / DYSPHAGIA
Nausea, vomiting, and early satiety can be symptoms caused by tumor compression and/or lymphadenopathy compressing the esophagus or diaphragm. Dysphagia (difficulty with swallowing) may occur in patients with lung cancer when there is pharyngeal and/or esophageal discomfort. Enlargement of the mediastinal lymph nodes that is often seen with lung cancer can also cause external compression of the esophagus, leading to dysphagia.
HOARSENESS
Hoarseness may be due to various causes in a patient with lung cancer. It could be due to metastasis with the lung neoplasm as the primary site. The symptom of vomiting can also cause severe irritation of the throat, resulting in hoarseness if the vomiting is frequent enough. Excessive coughing may also cause hoarseness. It can also be caused by compression on the recurrent laryngeal nerve.
NECK AND FACIAL SWELLING
Superior vena cava syndrome (SVCS) may occur from a right-sided lung or lymph node tumor that is compressing the vena cava. This can produce congestive pressure in the smaller veins feeding into the vena cava, leading to edema in the neck and face. This may be accompanied by a bluish-red skin tone, headache, dizziness, and altered consciousness. In extreme cases, SVCS is potentially fatal (ACS, 2021b).
Neck and facial swelling in a patient with lung cancer can also occur secondary to forceful and repeated vomiting.
CARDIAC INVOLVEMENT
Pericardial effusion is an abnormal collection of fluid in the pericardial sac. Fluid can build up slowly in the case of a lung tumor that is putting pressure on the pericardium. A large effusion can also compress the surrounding areas, causing decreased cardiac output. If the effusion causes compression on the lung tissue, cough, dyspnea, orthopnea, and tachypnea may result. Compression on the phrenic nerve may also cause a paralyzed diaphragm and shortness of breath. If the volume of the effusion is sufficient, there may be muffled or distant heart sounds, neck vein distension, peripheral edema, or a pericardial friction rub.
Cardiac tamponade results when a large volume of pericardial effusion causes compression directly on the heart. The onset occurs more slowly in the case of tamponade due to a slow-growing tumor. Mediastinal tumors that compress the heart may also causing dysrhythmias, particularly supraventricular tachycardias. In the case of lung cancer, cardiac dysrhythmias are a rare complication (Zaborowska-Szmit et al., 2020).
PLEURAL INVOLVEMENT
Malignant pleural effusion is the combination of excess pleural fluid combined with cancer cells in the pleural membranes. In the case of lung cancer, the tumor may block the pleural fluid from draining into the lymphatic circulation. The excess pleural fluid and malignant cells can cause pressure, leading to chest pain and shortness of breath (Harding et al., 2020).
There are two layers of pleura: the visceral pleura lines the lungs, and the parietal pleura covers the chest wall. Since there are no nerve endings or sensory pain fibers in the visceral pleura, any inflammation or disruption in the visceral pleura will not cause pain to the patient with lung cancer if that is where the tumor is located. The parietal pleural does have sensory nerve fibers and will be painful if there’s irritation or inflammation at this site.
PSYCHOSOCIAL ISSUES AND LUNG CANCER DIAGNOSIS
- Diagnosis with lung cancer can be a shock to the patient because of its 80%–90% mortality rate.
- Association with smoking or vaping as the cause of lung cancer may lead to the stigmatization of the patient.
- Depression is a common response to a lung cancer diagnosis, often caused by fear of the possible fatal outcome or the harsh effects of chemo- or radiation therapy.
- Anxiety initially interferes with an individual’s ability to understand patient education and to make self-care decisions.
(Bellomo et al., 2019)
Imaging Studies
Imaging studies are an essential aspect of diagnostic testing for lung cancer and used to determine tumor placement, lymph node involvement, and metastasis.
CHEST X-RAY
A chest X-ray is a common first diagnostic test to determine if a patient has lung cancer. The results may initially be normal, as lung tumors are slow growing. The X-ray will eventually show the location and the size of the lesion; any possible lymphatic, rib, or vertebral metastases; and any infiltrates or pleural effusions.
CT SCAN OF THE CHEST
Any lung mass sighted on X-ray will be further evaluated by a CT scan of the chest. This can be done without contrast dye. A more precise view of the location and size of the mass will be made available. Any mediastinal or lymph node enlargement will be more easily evaluated. CT scans of the brain, pelvis, and abdomen may also be performed to assess for metastasis.
OTHER SCANS
Additional scans may be used for diagnosis and/or staging a tumor:
- A positive emission tomography (PET) scan uses an intravenous radioactive dye as a tracer to locate tumors in organs or soft tissue. PET scans are considered 90% accurate compared to 75% accuracy for any other type of scanning.
- A magnetic resonance imagery (MRI) scan uses radio waves and magnets to visualize soft tissue for lung tumors and any metastases, including in the brain or spinal cord.
- Bone scans may be done to check for metastases to any bones or vertebrae if a PET scan or other scan identify any suspicious lytic or bone lesions.
(Harding et al., 2020)
(See also “Types and Staging of Lung Cancer” later in this course.)
Laboratory Findings
At this time, there is no lab test utilized specifically to diagnose lung cancer. However, some common laboratory tests may be included as part of a comprehensive assessment and might alert one to a possible diagnosis of lung cancer.
WHITE BLOOD COUNT (WBC)
A patient with lung cancer will exhibit a high white blood cell count in the presence of concurrent infections such as bronchitis and pneumonia. If systemic therapies (i.e., chemotherapy, immunotherapy, and targeted therapy) or radiation therapy are given as adjunct therapy for a patient with lung cancer, the treatments can affect the bone marrow, where blood cell are formed, causing a low white blood cell count (Healthline, 2021b).
MOLECULAR RESIDUAL DISEASE (MRD) ASSAY
MRD assay results are still considered somewhat experimental and not currently used as the standard of care. MRD measures circulating tumor DNA (ctDNA) that will indicate the presence of cancer cells, even after treatment. The presence of ctDNA is used for asymptomatic cancer screening, early cancer recurrence monitoring, scrutinizing the body’s response to treatment, and for the specific selection of treatments for the cancer (Natera, 2021).
ARTERIAL BLOOD GASES (ABGs)
An ABG test measures the acidity of blood (also referred to as pH) and the blood levels of oxygen (O2) and carbon dioxide (CO2) to determine the functional levels of air exchange. In a patient with lung cancer, ABGs may indicate compromised air exchange due to tumors. ABG results can also be used to determine the effectiveness of lung cancer treatments (CTCA, 2021).
Biopsy
A definitive diagnosis of lung cancer will be made by biopsy of a sample of the tumor tissue. This may be performed by aspiration, a lighted scope, or fluid collection assisted by ultrasound or video camera.
CT-GUIDED NEEDLE ASPIRATION
A needle biopsy may be performed using CT imaging or fluoroscopy to precisely place the needle in the presumed tumor in order to aspirate sufficient fluid or cells to be tested for malignancy. Contrast dye may or may not be used to better visualize the site of the tumor (Cedar Sanai, 2020b).
BRONCHOSCOPY
A biopsy performed by bronchoscopy requires that the patient be sedated. A physician will use a bronchoscope to visualize advancement to the area of the tumor in order to cut a small sample of tissue to send to the laboratory for biopsy. The tissue is either chemically treated or frozen, then sliced to be viewed under a microscope.
MEDIASTINAL EVALUATION
Mediastinoscopy
A mediastinoscopy with biopsy is a performed under general anesthesia and with an endoscopy tube in place to assist respirations. A small incision is made over the sternum and a tube inserted into the mediastinum in order to collect tissue samples to test the area of the mediastinum for cancer cells (MedlinePlus, 2020b).
Endobronchial Ultrasound with Biopsy (EBUS)
An EBUS is a minimally invasive procedure that is performed by aspiration to obtain liquid or tissue from the lung or lymph nodes in order to diagnose and stage lung cancer. An endoscope fitted with ultrasound and a fine-gauge aspiration needle is guided through the patient’s trachea. This procedure is speedy and accurate so that an onsite pathological diagnosis can be performed (USDH, 2021).
VIDEO-ASSISTED THORACOSCOPIC SURGERY (VATS)
A biopsy can also be obtained via video-assisted thoracoscopic surgery. This is a minimally invasive procedure performed under general anesthesia and with intubation to support breathing. One or more small incisions are made in the chest wall in order to insert a tiny camera (thoracoscope). The camera helps the physician to visualize the surrounding area in order to obtain tumor tissue to perform a biopsy to test for lung cancer, mesothelioma, or other chest cancers. The following are possible complications of the procedure:
- Pneumonia
- Bleeding
- Temporary or permanent nerve damage
- Damage to organs near the procedure site
- Anesthesia-related effects
(Mayo Clinic, 2020b)
When compared with a more complex, open operation such as a thoracotomy, VATS usually results in less pain, fewer complications, and a shorter recovery time, resulting in a hospital stay of only two to three days (Mayo Clinic, 2020b).
THORACENTESIS
A thoracentesis can be used to collect fluid to be tested for cancerous cells. A physician inserts a large needle and a catheter between the ribs and into the pleural space between the lung and the chest wall. Pleural fluid is aspirated for testing. Possible risks are pneumothorax, bleeding, or infection. A chest X-ray is ordered to be done immediately after the procedure to ensure there is no pneumothorax.
Pulmonary Function Testing (PFT)
Pulmonary function tests are useful diagnostic tools for lung cancer and its subsequent treatment. For instance, PFT results may indicate whether the lungs are sufficiently healthy for the patient to undergo systemic therapies (i.e., chemotherapy, immunotherapy, and targeted therapy) or radiation treatments. PFTs also establish a baseline of function for comparison prior to the patient starting treatment and subsequently to measure how treatment is affecting the lungs. PFT can also help establish whether the noncancerous lung will function adequately if the other one is removed (Canadian Cancer Society, 2021).
PFTs are noninvasive and are used to measure lung volume, capacity, rates of air flow, and gas exchange. The common method of measurement in pulmonary function tests is spirometry. This is performed by having the patient breathe into a mouthpiece connected to the electronic spirometer device to measure the rate and volume of respirations.
COMPONENTS OF PULMONARY FUNCTION TESTS
- Tidal volume (VT): volume of air inhaled or exhaled during normal breathing
- Minute volume (MV): volume of air exhaled over one minute
- Vital capacity (VC): volume of air that can be exhaled after inhaling as fully as possible
- Forced vital capacity (FVC): amount of air exhaled forcefully and quickly after inhaling as fully as possible
- Functional residual capacity (FRC): volume of air left in lungs after exhaling normally
- Residual volume: volume of air left in the lungs after exhaling as fully as possible
- Total lung capacity: volume of the lungs when filled with air as fully as possible
- Forced expiratory volume (FEV1): volume of air expired during the first second of the FVC test
- Forced expiratory flow (FEF): average rate of flow during the middle half of the FVC test
- Peak expiratory flow rate (PEFR): fastest rate one can force air out of the lungs
(JHM, 2020a)
The most significant finding related to the pulmonary tests is the FEV1/FVC ratio. A ratio less than 70% is considered indicative of substantial airway restriction.
Risks associated with pulmonary function tests are dizziness, shortness of breath, coughing, and asthma symptoms following deep breathing. Exclusion factors are recent eye, chest, or abdominal surgery; chest pain or a recent myocardial infarction; an aneurysm in the chest, abdomen, or brain; active tuberculosis; or any respiratory infection. The following would preclude a patient from having accurate PFTs: poor cooperation or effort, use of bronchodilators prior to testing, use of analgesics, pregnancy, bloated stomach, and fatigue.
Preparations for PFTs include cessation of the aforementioned medications, cessation of smoking for 8–24 hours as tolerated, and eating only a light meal just before the tests (JHM, 2020a).
PATIENT INSTRUCTIONS FOR PFTs
Prior to conducting a PFT, the clinician instructs the patient as follows:
- Empty your bladder before the testing begins.
- Loosen any tight-fitting clothing, jewelry, or other items that may interfere with the test.
- Remove your dentures, if applicable.
- Sit in a chair.
- A soft clip will be put on your nose so that all of your breathing is done through your mouth, not your nose.
- You will be given a sterile mouthpiece attached to a spirometer. Form a tight seal over the mouthpiece with your mouth.
- Follow the clinician’s instructions to inhale and exhale in different ways.
- You will be monitored carefully during the test for dizziness, trouble breathing, or other problems.
- You may be given a bronchodilator drug after certain tests. The tests will then be repeated several minutes later, after the bronchodilator has taken effect.
(URMC, 2020)
LUNG CANCER DIAGNOSIS AND COVID-19
Diagnosis and treatment of patients with lung cancer has become more difficult as a result of the COVID-19 pandemic. This is due in part to the fact that standard physiologic and staging assessments for lung cancer—such as pulmonary function testing (PFTs), endobronchial ultrasound, and bronchoscopy—are droplet-producing and aerosolizing procedures that place healthcare personnel at risk and may therefore reduce the use of such procedures.
Likewise, COVID-19 and lung cancer symptoms are similar (cough, shortness of breath, fatigue, congestion, or pain or pressure in the chest), which may delay the diagnosis of lung cancer.
TYPES AND STAGING OF LUNG CANCER
There are several different types of lung cancer and various staging systems, as described below. Treatment may be determined by the type and stage that is given to the tumor when the cancer is diagnosed.
Primary Lung Cancer
Together, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) account for 95% of all lung tumors. These two types of tumors are malignant in character, whereas the remaining 5% of lung tumors may be malignant or benign.
NON-SMALL CELL LUNG CANCER
Squamous Cell Carcinoma
- 20%–30% of all lung cancers
- Centrally located
- Produces early symptoms of nonproductive cough and hemoptysis
- Does not tend to metastasize
- Depending on stage at diagnosis, may be treated by surgical resection, radiation, and/or systemic therapies
- Depending on the staging upon diagnosis, life expectancy tends to be better than with small cell lung cancer
- Slow growth rate
Adenocarcinoma
- 30%–40% of all lung cancers
- Most common lung cancer in nonsmokers
- Peripherally located
- Usually no symptoms until there is considerable metastasis
- Depending on the staging upon diagnosis, may be treated by surgical resection, radiation, and/or systemic therapies
- Moderate growth rate
Large Cell (Undifferentiated) Carcinoma
- 10% of all lung cancers
- Large anaplastic cells
- Often located in the bronchi
- Highly metastatic via blood and lymphatic system
- Surgery not usually a credible option because of the high rate of metastasis to other organs
- Tumor may respond well to radiation therapy but has a high rate of recurring
- Rapid growth rate
SMALL CELL CARCINOMA
- <20% of all lung cancers
- Most malignant type of lung cancer
- Early metastasis via blood and lymphatic system
- Frequently metastasizes to the brain
- Treated with systemic therapies, but the prognosis is still usually poor
- Radiation therapy may be used in addition to systemic therapies or as a palliative measure to reduce symptoms
- Is usually not considered resectable as it is so fast-growing
- Related to endocrine disorders
Other Lung Tumors
MESOTHELIOMAS
- May be malignant or benign tumors
- Malignant tumors are related to exposure to asbestos
- Arise from the visceral pleura
- Benign mesotheliomas are localized lesions
HAMARTOMAS
- Most common type of benign lung tumor
- Slow-growing type of congenital tumor
- Composed of fibrous tissue, fat, and blood vessels
MUCOUS GLAND ADENOMA
- Benign tumor in the bronchi
- Composed of columnar cystic spaces
(Harding et al., 2020)
Staging Non-Small Cell Lung Cancer
Following diagnosis with lung cancer, clinicians describe the extent of the cancer by designating its “stage.” Staging is then used in planning appropriate interventions. Patients with NSCLC in stages I, II, and IIA are viable candidates for surgery since their malignancy is not too advanced. Patients with stages IIIB and IV cancer are inoperable and have a poor prognosis (Harding et al., 2020).
| (Harding et al., 2020) | |
| Stage I | Tumor is small and localized to the lung, with no lymph node involvement |
| A | Tumor <3 cm |
| B | Tumor 3–5 cm and invading surrounding local areas |
| Stage II | Increased tumor size, some lymph node involvement |
| A | Tumor 3–5 cm with lymph node involvement on same side of chest OR Tumor 5–7 cm without lymph node involvement |
| B | Tumor 5–7 cm involving the bronchus and lymph nodes on the same side of the chest and tissue of other local organs OR Tumor >7 cm without lymph node involvement |
| Stage III | Increased spread of tumor |
| A | Tumor spread to the nearby structures (chest wall, pleura, pericardium) and regional lymph nodes |
| B | Extensive tumor involving heart, trachea, esophagus, mediastinum, malignant pleural effusion, contralateral lymph nodes, scalene or supraclavicular lymph nodes |
| Stage IV | Distant metastasis |
The TNM tumor classification system further evaluates tumors according to tumor size and invasiveness (T), regional spread to the lymph nodes (N), and metastasis (M). These designations are used for both SCLC and NSCLC, although it is generally not as important for SCLC because this cancer is aggressive and systemic. The stages of SCLC are limited, as the tumor is only on one side of the chest and an extensive disease.
| (Lewis et al., 2020) | |
| Primary Tumor (T) | |
|---|---|
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ (IS) |
| T1–T4 | Ascending degrees of increase in tumor size and involvement |
| Tx | Tumor cannot be measured or found |
| Regional Lymph Nodes (N) | |
| N0 | No evidence of disease in lymph nodes |
| N1–N4 | Ascending degrees of nodal involvement |
| Nx | Regional lymph nodes unable to be assessed clinically |
| Distant Metastases (M) | |
| M0 | No evidence of distant metastasis |
| M1–M4 | Ascending degrees of metastatic involvement, including distant nodes |
| Mx | Cannot be determined |
(See also “Resources” at the end of this course.)
LUNG CANCER TREATMENT MODALITIES
There are various treatment modalities for lung cancer. Selection of which treatment or combination of treatments to be used is made by the patient together with the patient’s oncologist, other physicians, and other healthcare professionals.
Surgery
Small cell lung cancer (SCLC) is not generally treated by surgery because the cancer is so rapid and diffuse in its growth.
If a malignant lung tumor is considered operable, several cardiopulmonary evaluations must be done before the surgery can go forward. Pulmonary function studies, arterial blood gases (ABGs), an electrocardiogram (ECG), a complete blood count (CBC) and metabolic panel, and an anesthesia consult must all be performed to assess the cardiopulmonary status of the prospective surgical patient (Harding et al., 2020).
A possible contraindication for the surgical treatment of lung cancer is advanced lung disease such as chronic obstructive pulmonary disease (COPD). A patient with advanced COPD may have insufficient lung capacity to be weaned off the ventilator. Once this patient has demonstrated ventilator dependence, there may not be an opportunity to breathe on their own once they are extubated.
Any lung cancer surgery is a highly invasive procedure for the older adults >70 years of age, with higher rates of morbidity and mortality than for younger patients. With the advent of less invasive surgery, such as video- or robot-assisted thoracoscopy (VAT or RAT), morbidity and mortality rates have improved because of a reduction in cardiopulmonary complications (Zaatar et al., 2020).
(See also “Prehabilitation” below.)
PULMONARY COMORBIDITIES AND LUNG SURGERY
Patients with pulmonary comorbidities such as COPD and asthma will fare better with resections that consist of shorter surgical and anesthesia times, and therefore shorter intubation times. Many patients with this level of lung disease will not be considered good surgical candidates when an analysis of the probability of extubating the patient is performed by the prospective surgeon and anesthesiologist.
TYPES OF SURGERY
The type of lung resection is determined by the cancer stage at initial presentation and the mass’s size, location, and proximity to adjacent anatomy. Different surgical approaches can be used for lobectomy, segmentectomy, sleeve resection, and mediastinal tumor resection.
Pneumonectomy
A pneumonectomy is the most radical lung surgery and involves excising the entire lung. The first pneumonectomy was documented in the Journal of the American Medical Association (JAMA) in 1933. The extent of this surgery for NSCLC may include the anatomic resection of hemipulmonary tissue and pulmonary artery, vein, and main bronchus, as well as lymph node dissection if there is metastasis to the lymph nodes.
A pneumonectomy may be chosen for treatment of lung cancer given the size and location of the tumor, for example if the tumor is in the center of the chest or an advanced mesothelioma. The 5-year overall survival (OS) rate is 32.3%, and the 30-day mortality rate is 4%.
Lobectomy
Lobectomies are considered the “gold standard” or optimum lung surgeries for treatment of early-stage (stages I–IIA) NSCLC. In this surgery, a single lobe of the left lung or one or two lobes of the right lung are removed. A sleeve lobectomy includes also removing part of the near bronchus. Pulmonary function tests are often performed to determine whether the patient will be able to tolerate a traditional open lobectomy or whether a less-invasive, video-assisted thoracoscopic surgery (VATS) would be the better choice (see below).
Wedge Resection and Segmentectomy
Wedge resection (also called sublobar resection) and segmentectomy (or segmental resection) lung surgeries are two surgical options for high-risk but operable patients with early-stage (stages I or II) lung cancer. Wedge resection or segmentectomy are considered good procedures for borderline surgical candidates with limited pulmonary reserve, such as those with pulmonary comorbidities such as emphysema or interstitial lung disease (ILD) (Kawaguchi et al., 2019).
A wedge resection involves removal of the tumor plus a wedge-shaped section of lung tissue around the tumor to ensure clear surgical margins. A segmentectomy surgery involves removal of only part of a lobe of the lung. Wedge resection or segmentectomy are connected with fewer peri- and postoperative complications, but the incidence of recurrent cancerous lung tumors is higher than among patients who receive a standard lobectomy.
Video-Assisted Thoracic Surgery (VATS)
VATS is a minimally invasive technique for diagnostic and surgical resection of a lung tumor, usually one near the outside of the lung. It is the recommended approach for a stage I NSCLC tumor. Small incisions are placed in the chest wall in order to insert a tiny camera to guide the procedure. Surgical instruments are then inserted through the small incisions to excise the targeted lung tumor.
The use of VATS rather than more traditional and more extensive lung surgery has the benefits of less pain, superior postoperative shoulder range of motion (ROM), and better general function immediately postoperatively. There are fewer postoperative complications, a significantly diminished risk of intensive care readmission, shorter hospital lengths of stay, and diminished necessity for rehabilitation while still in the hospital while recovering from surgery (Al-Ameri et al., 2019).
Robotic-Assisted Thoracic Surgery (RATS)
RATS is being performed more frequently than VATS for diagnosis and lung resections. There are several advantages of using RATS, including a magnified three-dimensional view, a manipulator wrist with better dexterity, and a tremor filtration mechanism. Four to five incisions are necessary for the various arms used in RATS, compared to one incision with VATS. Three-dimensional (3D) video and surgical scopes are then inserted through the incisions. The robotic arms move as flexibly as a human hand and wrist, manipulated by the surgeon from the console in the room.
The use of robotic equipment for a lobectomy increased from <1% in the United States in 2009 to an estimated >20% in 2020. The complexity of lung resections performed by RATS has increased as well (Mazzei & Abbas, 2020). It is primarily used for stage I/II NSCLC.
Robotic segmentectomy is more complex than robotic lobectomy, depending on the ability to access the tumor with the robotic arms. Robotic bronchial sleeve resection is the most difficult of the robotic-assisted lung surgeries. The tumor is excised and then the bronchial tissue is re-anastomosed. The postoperative complication rate is lower than with traditional lung resections (Han et al., 2020).
SURGERY AND COVID-19 CONCERNS
The high transmissibility of the virus that causes COVID-19 has introduced new concerns in regard to lung cancer surgery. For instance, the operative team may face exposure risk to aerosolized viral load during endotracheal tube placement or any type of airway surgery. Operative time may thus be increased or the availability of operating rooms decreased due to changed anesthesia protocols caused by COVID precautions.
A group of experts from Switzerland have developed an algorithm to separate patients with lung cancer into categories of surgical urgency. Patients with a high risk of disease progression and a low risk of COVID infection are recommended to undergo definitive oncological treatment (Hilzenrat et al., 2021).
ADVERSE EFFECTS
The adverse effects of lung surgery are similar to those of any surgery. Hemorrhage intra- and postoperatively is the most dangerous occurrence, possibly due to a slipped ligature. Pre- and postoperative blood counts need to be carefully monitored for this reason.
Depending on the size of the excised lung tissue, the incision can be quite extensive. Posterior-lateral incisions provide the best access but can be very painful. The incision may involve dividing latissimus dorsi, serratus anterior, and trapezius muscles. The resultant pain can cause the patient to hypoventilate, resulting in hypercapnia, atelectasis, or postoperative pneumonia. An air leak intra- or postoperatively can cause pneumothorax, requiring the insertion of a chest tube. A chest tube may also be inserted to measure blood loss or to equalize intrathoracic pressure changes.
Other possible postoperative complications include:
- Pain due to the surgery; treated by opiates immediately postoperatively
- Persistent air leak caused by trauma to the pleura during surgery; treated by fibrin glue, early pleurodesis (adhesion of pleura to the chest wall), a blood patch, or dissection of the visceral pleura
- Atrial fibrillation or other supraventricular dysrhythmias
- Chylothorax (lymph fluid from the digestive system that can migrate to the chest cavity)
(Nakamura et al., 2021)
POSTOPERATIVE INFECTIONS
Postoperative surgical-site infection (SSI) is a common side effect of any surgery, including lung cancer surgery. Patients who undergo surgery for NSCLC are also at high risk for a postoperative pneumonia, which is much more common and potentially fatal. Medicating the patient prophylactically with antibiotics has proved to be effective in preventing postoperative infection.
Patients who are immunosuppressed by having glucocorticoid steroid therapy to alleviate adverse symptoms from the lung cancer or systemic therapies preoperatively are at even higher risk for postoperative infections. Cephalosporins given preoperatively for prophylaxis have been effective in preventing SSIs. Sulbactam/ampicillin (Unasyn) intravenously has proved to be highly effective in preventing pneumonitis in postoperative lung surgery.
Other factors that have shown to reduce postoperative pneumonia are preoperative pulmonary rehabilitation, extensive preoperative oral hygiene, and comprehensive perioperative respiratory care (Deguchi et al., 2019).
PREHABILITATION
Prehabilitation is a relatively new treatment method used preoperatively to assess whether a patient will tolerate surgery and to maximize their physical status prior to surgery. Prehabilitation prepares the patient physiologically and psychologically in order to promote better postoperative outcomes, prevent untoward effects of extensive surgery, improve quality of life, and reduce postoperative morbidity (Lai et al., 2017). Prehabilitation includes interventions in the areas of physical therapy, occupational therapy, and pulmonary rehabilitation.
Before a comprehensive rehabilitation treatment plan can be formulated, it is necessary for the clinician to conduct a comprehensive physical and pulmonary assessment, including vital signs, oxygen saturation, height, weight, and pulmonary function testing (JHM, 2020a).
Functional capacity measures in particular are strong predictors of postoperative complications such as respiratory failure, increased hospital length of stay, and health-related quality of life. These measures are also able to predict postoperative mortality and long-term survival in NSCLC. Testing to determine a prospective surgical patient’s fitness for the operation may include the 6-minute walking distance (6-MWD), peak expiratory flow (PEF), and quality-of-life scores (Lai et al., 2017).
The 6-MWD test is a means to measure a patient’s stamina, endurance, and potential survival. The parameters measured are oxygen saturation, pulse, and a subjective description of the patient’s level of dyspnea. A significant improvement in the 6-MWD measured after a lung resection surgery compared to the prehabilitation measurement proves to be a credible predictor of improvements in morbidity and mortality postoperatively (MDApp, 2020).
Prehabilitation interventions such as aerobic exercise and resistance training may improve physical and pulmonary status in patients considered to be poor surgical candidates due to cardiovascular or pulmonary impairment. This may lead such patients to become candidates for surgical resection. Interventions are supervised, outpatient-based, and typically performed five times a week for one to 10 weeks (most commonly, for four weeks).
Exercise training performed preoperatively has been found to improve patient outcomes for patients who undergo surgery for lung cancer. Exercises taught to the patient preoperatively focus on endurance and resistance training. Aerobic training is considered the best way to improve cardiopulmonary fitness, and when part of a pulmonary rehabilitation program, aerobic training has proven to reduce dyspnea, increase functional capacity, and reduce postoperative morbidity. Other prehabilitation interventions may include breathing exercises, incentive spirometry, inspiratory muscle training, stretching, and relaxation.
POSTOPERATIVE MANAGEMENT
Physical therapists play a significant role in postoperative patient care in the form of enhanced recovery after surgery (ERAS), and for longer-term pulmonary rehabilitation. Postoperative physical therapy management includes breathing exercises for pulmonary expansion, inspiratory muscle training (IMT), bronchial hygiene, early mobilization and ambulation, postural correction, and surgical-side shoulder range of motion (ROM) as a recovery and maintenance strategy.
IMT improves inspiratory muscle strength and endurance, functional exercise capacity, dyspnea, and quality of life. Pulmonary muscle weakness is a critical impairment following pulmonary surgery, secondary to muscular injury, central nervous system depression, and pain. Pulmonary muscle weakness causes the inability to cough effectively, reduced lung compliance, and dyspnea related to postoperative immobilization.
In order to avoid atelectasis (lung collapse or closure) and to prevent other postoperative complications, it is imperative to remove secretions from the airways and promote expansion of the lung tissue. Deep-breathing exercises with bronchial clearance, exercising and stretching the surgical-side muscles, and early patient mobilization are essential aspects of the postoperative pulmonary physical therapy program (Kendall et al., 2017).
The respiratory therapist (RT) will manage the patient on a mechanical ventilator immediately after tumor resection surgery or a pneumonectomy and make recommendations for weaning the patient off of the ventilator and extubating the patient. It is the RT who determines the type of oxygen delivery system: nasal cannula, simple mask, Venturimask, tracheostomy collar, or a nonrebreather mask. The RT may draw the arterial blood gases (ABGs) to determine the amount of oxygen needed to be delivered to the patient.
To maintain a clear airway, the RT will give the patient nebulizer treatments and instruct them on how to use medi-halers and discs. It is also the responsibility of the RT to suction the patient’s airway oral pharyngeally, nasal pharyngeally, and tracheally. The RT will also perform chest percussion and provide instruction in handling and removing secretions to promote as much independence during the palliative phase (Harding et al., 2020).
Radiation Therapy
Radiation therapy may be used to treat lung cancer depending on the stage, size, or progression of the tumor and other factors:
- As the main treatment, in conjunction with systemic therapies, if the tumor is inoperable because of a large size or difficult location or if a patient is either a poor candidate for surgery or declines surgery
- Preoperatively, with systemic therapies, to shrink the tumor to facilitate excision
- Postoperatively for thorough eradication of the malignant cells
- To treat metastasis when the lung cancer is the primary site
- To relieve symptoms such as pain, dysphagia, or cough
Side effects of radiation therapy for lung cancer include fatigue; nausea and vomiting; loss of appetite and/or weight loss; skin changes in the area being treated, ranging from redness to blistering and peeling; and hair loss at the site of radiation. Side effects depend on the dose and duration of the treatments. The side effects may be minimal, allowing the patient to continue daily functions, or much more severe. They may go away after treatment. The combination of radiation therapy given with systemic therapies may result in worse side effects (ACS, 2020a).
EXTERNAL BEAM RADIATION THERAPY (EBRT)
In EBRT, high-dose beams of radiation are focused on the tumor utilizing a special machine called a linear accelerator. The machine moves around the body without touching the patient and can deliver high-energy radiation beams to a tumor from any angle and shaped to the contour of the tumor. Targeting a tumor with higher, more precise doses of radiation can reduce damage to healthy tissue and nearby organs. As a result, EBRT may also help reduce the risk of side effects associated with traditional radiation treatment (NCI, 2020c).
Stereotactic Body Radiotherapy (SBRT)
Stereotactic body radiotherapy, also referred to as stereotactic ablative radiotherapy (SABR), is a form of EBRT. It involves several high-dose beams of radiation focused on the tumor from different angles to target the tumor precisely in order to minimize damage to surrounding tissue and extends for one to five treatments. SBRT is used for early-stage lung cancer that may be inoperable due to age, health problems, or location of tumor; when the patient rejects surgery; or in cases with limited metastasis to other organs (ACS, 2020a).
The precisely focused high-dose radiation beams use 3D imaging as a noninvasive means to treat a tumor and prevent harm to the surrounding tissue. This can be performed by using a linear accelerator or proton beam therapy, which requires only one to five treatment sessions, depending on the size of the tumor. Early side effects are usually temporary and may include fatigue, swelling, and nausea and vomiting. Late side effects are rare and may occur months after the treatment(s). These may include weakened or broken bones, bowel changes, changes in the lungs, changes in the spine, a secondary cancer, or lymphedema (Mayo Clinic, 2022).
Three-Dimensional Conformal Radiation Therapy (3D CRT)
Three-dimensional conformal radiation therapy is a form of EBRT that uses a computer to precisely determine the location of the tumor. Radiation beams are shaped and delivered in multiple angles to focus on the tumor and preserve the surrounding lung tissue, protecting it from the radiation.
Intensity-Modulated Radiation Therapy (IMRT)
Intensity-modulated radiation therapy is a type of 3D radiation therapy that also uses a computer to shape the beams of radiation and direct them at the lung tumor from various angles. In this type of radiation therapy, the intensity of the beams can be adjusted to help preserve the surrounding lung tissue. Volumetric modulated arc therapy (VMAT) is another variation in which a device rotates quickly around the body and aims a precise dose at the tumor in just a few minutes.
PROPHYLACTIC CRANIAL RADIATION
Prophylactic cranial radiation is used for SCLC with early metastasis to the central nervous system (CNS). It decreases the instances of cerebral metastases and improves the survival rate in limited SCLC. Since most chemotherapies are unable to penetrate to the blood-brain barrier, prophylactic cranial radiation is given to treat metastasis to the brain from a primary site. The purpose is to improve the patient’s overall survival rate (Harding et al., 2020).
BRACHYTHERAPY
Brachytherapy treats cancer by implanting radioactive seeds, ribbons, rods, wires, or capsules into the interstitium of the tumor tissue to destroy the abnormal cancer cells directly and with minimal destruction of the surrounding tissue. The radioactive material is injected into the tumor via a catheter or applicator tube.
- A low-dose radiation treatment remains implanted for one to seven days.
- A high-dose radiation treatment is left in place for 10 to 20 minutes at a time and then removed. The treatment may also be given twice a day for two to five days, or once a week for two to five weeks.
- Permanent implants remain in place for the life of the patient, and the radiation gradually weakens.
A patient receiving brachytherapy is placed in a private room to prevent exposure from the radiation to others. Clinicians provide care in short intervals to minimize exposure to themselves. The following are the considerations for visitors to the patient during this time period:
- Not being allowed to visit when the radiation is first put in
- Needing to check with the hospital staff before visiting
- Standing by the doorway rather than going into the patient’s room
- Keeping visits short (30 minutes or less each day), depending on the type of radiation being used and the part of the body being treated
- Not having visits from pregnant women and children younger than 1 year
(NCI, 2020b)
The side effects of brachytherapy are diarrhea, nausea, skin irritation, fatigue, and dysuria.
RESPIRATORY-GATED RADIATION THERAPY
Respiratory-gated radiation therapy is a very new treatment that uses the period of time when the lungs are relatively immobile between respirations to administer the radiation therapy. This method allows the radiation to be administered more precisely to the tumor, preserving more of the surrounding healthy lung tissue (Dartmouth, 2020).
Chemotherapy
Chemotherapy is used in most cases of SCLC, as these patients are not considered candidates for surgical intervention. Some patients with NSCLC may also require chemotherapy depending on the stage of their cancer. Chemotherapy is also utilized as an adjuvant to other therapies (ACS, 2020b).
Patients in poor health may not be candidates for intense doses of chemotherapy or a combination of drugs due to tolerance, but older age itself is not a contraindication for chemotherapy.
- Chemotherapy may be utilized along with radiation therapy as the main treatment for locally advanced NSCLC cancers that have grown into nearby structures such that surgery is not an option or for people who are not healthy enough for surgery.
- Chemotherapy may be given for metastatic (stage IV) NSCLC that has spread to areas outside of the lungs, including bones, liver, or adrenal gland.
- Neoadjuvant (before surgery) chemotherapy may be used (sometimes with radiation therapy) to shrink a tumor in order to remove it with less-extensive surgery.
- Adjuvant (after surgery) chemotherapy may be used (sometimes with radiation therapy) to kill any cancer cells that might have been left behind or have spread but cannot be seen on imaging tests.
(ACS, 2020b)
CHEMOTHERAPY ADMINISTRATION
Chemotherapy drugs for lung cancer are usually given intravenously (IV), either as a slow IV push or as a “piggyback” infusion intermittently over a longer period of time. They can also be administered orally if nausea and malabsorption are not issues for the patient.
Administration can be performed in a physician’s office, chemotherapy clinic, or the hospital. Long-term chemotherapy may require the placement of a device left in place in a large vein, such as a peripherally inserted central catheter (PICC) or a vascular access device (VAD). Patients in poor health may not be able to tolerate higher doses of chemotherapy or a combination of drugs.
Chemotherapy is given in cycles, with each period of treatment followed by a rest period to allow recovery from the side effects of the drug(s). Cycles are usually three or four weeks in length. The schedule depends on the type of chemotherapy being given. With some drugs, the chemotherapy is given just on the first day of the cycle. Other chemotherapy is given for a few days in a row or weekly. Then the cycle repeats.
Adjuvant and neoadjuvant chemotherapy are given for three to four months, depending on the drugs that are ordered. The length of treatment for advanced lung cancer is based on the effectiveness of the treatment regimen and side effects.
For late-stage or extensive lung cancers, the initial chemotherapy combination is usually given for four to six treatment cycles. Often it is recommended to give the chemotherapy treatment for longer with a single chemotherapy or targeted drug.
Patients who have a good response to their initial chemotherapy or no worsening of their cancer may receive maintenance chemotherapy for a more prolonged period of time. Maintenance therapy can prevent or delay the return of the cancer if the patient is in remission, thus extending life expectancy (ACS, 2020b).
Combinations of two or more chemotherapy drugs are often used to treat early-stage lung cancer. Late-stage lung cancer may be treated at first by a combination of different chemotherapy drugs, such as gemcitabine with vinorelbine or paclitaxel, but the treatment regimen may be switched to a single drug such as docetaxel or pemtrexed to focus on the tumor(s). This may be done for patients who find combination chemotherapy intolerable, such as those in poor general health or of advanced age. For some people with late-stage lung cancer, a targeted therapy drug or an immunotherapy drug may be given along with chemotherapy (NCCN, 2021).
TYPES OF CHEMOTHERAPY
Platinum-based chemotherapy is considered the “gold standard” for the treatment of NSCLC because of its effectiveness in reducing the size of the tumor. It may be given prior to surgery.
Cisplatin is a platinum-based chemotherapy that acts as the primary treatment for advanced NSCLC, including squamous cell carcinoma, adenocarcinomas, adenosquamous carcinoma, large cell carcinoma, and sarcomatoid carcinoma. This may be combined with vinorelbine, gemcitabine, docetaxel, or pemetrexed to increase the efficacy of the drug.
Carboplatin is a platinum-based chemotherapy that can be used for chemotherapy treatment in patients with complicating comorbidities or in patients who are unable to tolerate cisplatin for a variety of reasons.
Other chemotherapy drugs used include:
- Paclitaxel (Taxol)
- Albumin-bound paclitaxel (nab-paclitaxel, Abraxane)
- Docetaxel (Taxotere)
- Gemcitabine (Gemzar)
- Vinorelbine (Navelbine)
- Etoposide (VP-16)
- Pemetrexed (Alimta)
CHEMOTHERAPY SIDE EFFECTS
Chemotherapy can cause many and severe side effects or adverse reactions. (The same side effects can also be caused by physiologic placement of the mass or lymphadenopathy compressing the esophagus or the diaphragm.) Some common side effects include:
- Nausea and vomiting
- Dysphagia
- Hair loss
- Mouth sores
- Loss of appetite or weight changes
- Diarrhea or constipation
Nausea and vomiting may happen within one hour of chemotherapy (or a few hours after radiation to the chest or abdomen). The duration of vomiting may persist for up to 24 hours after treatment.
Fatigue can either be caused by the cancer itself or as a side effect of chemotherapy. After treatment, accumulation of toxic substances may remain in the body, thereby causing fatigue.
Chemotherapy can also affect the blood-forming cells of the bone marrow, which can lead to:
- Increased chance of infections (from low white blood cell counts)
- Easy bruising or bleeding (from low blood platelet counts)
- Fatigue (from anemia secondary to low red blood cell counts)
Some drugs, such as cisplatin, vinorelbine, docetaxel, or paclitaxel, can cause peripheral neuropathy (ACS, 2020b).
Weight loss in patients with cancer may be due to pain causing a loss of appetite (anorexia), fatigue precluding the patient from preparing meals or taking the time to eat (cachexia), and dysphagia. At diagnosis, approximately 60% of patients with lung cancer have already experienced substantial weight loss (Chandrasekar et al., 2016).
Anorexia (loss of appetite) may result from the cancer itself or the treatment(s). Anorexia peaks at approximately four weeks into chemotherapy or radiation treatments and then resolves. Precautions must be taken to prevent weight loss and dehydration. It may become necessary to replace eating with enteral or parenteral nutrition.
Difficulty chewing or swallowing may result from an inflamed mouth or esophagus caused by chemo- or radiation therapy. As with nausea and vomiting, and anorexia, this symptom can interfere with the patient’s ability to ingest adequate nutrition. In this instance, the patient may indicate a lump as they swallow or that food gets stuck when they are eating (Harding et al., 2020).
Targeted Therapy
Targeted therapy uses drugs to inhibit the growth of the malignant molecules rather than kill cancer cells. It may be less toxic than chemotherapy or may be given in conjunction with chemotherapy or other lung cancer treatments.
The most common side effects seen with targeted therapies are diarrhea and liver problems, such as hepatitis and elevated liver enzymes. Other side effects seen with targeted therapies include:
- Skin problems (acneiform rash, dry skin, nail changes, hair depigmentation)
- Problems with blood clotting and wound healing
- High blood pressure
- Gastrointestinal perforation (a rare side effect of some targeted therapies)
(NCI, 2021)
Indications for the use of targeted therapy include:
- Epidermal growth factor receptor (EGFR) gene mutations
- Anaplastic lymphoma kinase (ALK) rearrangements
- ROS1 rearrangements
- Mesenchymal epithelial transition factor (MET) amplifications
- V-Raf murine sarcoma viral oncogene homolog B1(BRAF) mutations
TYROSINE KINASE INHIBITORS
Tyrosine kinase inhibitors block signals for growth in the cancer cells. Tyrosine kinase is an enzyme that speeds up molecular growth. Blocking its usual function slows the malignant cell growth. They can be used in patients with EGFR mutations.
Examples include:
- Cetuximab (Erbitux)
- Erlotinib (Tarceva)
- Afatinib (Gilotrif)
- Gefitinib (Iressa)
- Osimertinib (Tagrisso)
- Necitumumab (Portrazza)
(Harding et al., 2020)
KINASE INHIBITORS / ALK INHIBITORS
Another variety of kinase inhibitor is given to patients with NSCLC with an abnormal anaplastic lymphoma kinase (ALK) gene. These drugs inhibit the kinase protein manufactured by the ALK gene that causes cancer cell growth and development. Examples include:
- Crizotinib (Xalkori)
- Ceritinib (Zykadia)
(Harding et al., 2020)
ANGIOGENESIS INHIBITORS
Angiogenesis inhibitors slow the growth of new blood vessels by targeting the vascular endothelial growth factor. This inhibits the growth of a tumor by denying it an adequate blood supply. Examples include:
- Bevacizumab (Avastin)
- Ramucirumab (Cyramza)
Immunotherapy
Immunotherapy therapy boosts the immune response caused by the body to fight cancer cells. It targets the PD-1/PD-L1 protein on T cells that keeps them from attacking other cells. Immunotherapy drugs can shrink some tumors to prepare for excision or alleviate symptoms or slow their growth. The drugs also effectively treat metastatic NSCLC that continues to grow after other treatments have been tried. Tumors that actively express PD-1/PD-L1 are also treated by these targeted drugs. Examples include:
- Nivolumab (Opdivo)
- Pembrolizumab (Keytruda)
- Atezolizumab (TelCentris)
- Durvalumab (Imfinzi)
(Harding et al., 2020; Niu et al., 2021)
Similar to PD-1/PD-L1 inhibitors, CTLA-4 inhibitors boost the immune response to fight cancer and have a similar mechanism of action to PD-1/PD-L1 inhibitors. An example is:
- Ipilimumab (Yervoy)
(ACS, 2021c)
Immunotherapy for lung cancer may cause side effects, usually related to inflammation, such as:
- Pneumonitis
- Fatigue
- Cough
- Shortness of breath
- Nausea
- Anorexia
- Diarrhea
- Muscle and bone pain
(ALA, 2020c)
Photodynamic Therapy (PDT)
Photodynamic therapy is a two-stage treatment that combines light energy with a photosynthesizer drug. It can be used in early-stage cancers that have not metastasized in order to destroy cancerous cells that are easy to reach. The photosynthesizer drug is given intravenously and concentrates around the tumors in the esophagus and outer airway. After 48 hours a bronchoscopy is performed to apply a laser to the targeted areas, which activates the drug, causing cell death. Another bronchoscopy is performed a few days later to remove necrotic tissue. PDT can cause the cancerous tissue to “starve” and the immune system to fight the cancer cells. The most commonly used photosynthsizer drug is porfimer (Photofrin) (Harding et al., 2020).
The most common side effect of PDT is sensitivity in the patient to bright lights and sunlight. Reactions caused by PDT light can show up on the skin where the drug is applied. They usually involve redness and a tingling or burning sensation. Interventions include:
- Staying out of strong, direct light
- Staying indoors as much as possible
- Wearing protective clothing and wide-brimmed hats to avoid sunlight when outdoors
- Avoiding beaches, snow, light-colored concrete, or other surfaces where strong light may be reflected
Sunscreens will not protect the skin from photosensitivity reactions (ACS, 2020a).
Radiofrequency Ablation
Radiofrequency ablation treats small NSCLC near the outer edge of the lungs. It is used as an alternative treatment method when surgery is either not possible or not desired. A needle is passed into the tumor through the skin under CT visualization. High-energy radio waves create an electric current that passes through the needle, heating and destroying the cancerous cells. Side effects are mild and may include numbness, weakness, soreness, or itching (Harding et al., 2020).
Lung Cancer Symptom Treatments
Symptoms of lung cancer are related to the type, size, location, and extensiveness of the disease. Managing a patient’s symptoms can lead to better patient outcomes. For patients with primary lung cancers confined within the lung(s), symptoms are usually related to issues with the respiratory tract (see “Clinical Manifestations” earlier in the course).
Patients who have lung cancer that has spread to other parts of the body may have a variety of symptoms. For example, patients with metastatic disease in the brain (a common site of metastasis) may have headaches, vision impairment, personality changes, or other neurological deficits. Additionally, patients with metastatic disease to the bone often have significant pain and are at higher risk of bone fractures (also called pathological fractures). (Considerations for patients with different metastatic disease sites are also discussed later in this course.)
BRONCHODILATORS
Bronchodilators are given to patients with lung cancer to increase the volume in the airway that may be obstructed due to swelling, excessive secretions, or a tracheal or bronchial tumor. Several types of bronchodilators can serve this purpose by reducing the obstruction. Use of bronchodilators in patients with lung cancer has been measured to significantly increase the forced exhaled volume (FEV) (Ueda et al., 2018).
The two most common classifications of bronchodilators used for lung cancer include:
- Beta-2 agonists (salbutamol, salmeterol, and formoterol)
- Anticholinergics (ipratropium, tiotropium, and glycopyrronium)
The route of administration is inhaled via nebulizer or a powder disc. Common side effects are shakiness, tachycardia, palpitations, dyspepsia, insomnia, and muscle cramps.
MUCOLYTIC AGENTS
Mucolytic agents are used to reduce the viscosity of unusually thick respiratory secretions in order to promote improved expectoration of the sputum. Patients with lung cancer frequently produce secretions that are excessively thick or purulent. Effective use of mucolytics reduces the amount of a patient’s disability, reduces the hospitalization length of stay, causes respirations to ease, and improves quality of life.
Examples of mucolytic agents include:
- N-acetylcysteine
- Carbocysteine
- Erdosteine
- Ambroxol
The route of administration is oral (tablets and liquid) or nebulization. Side effects may include nausea, sore throat, nasal drainage, white patches on the lips and mouth, and diarrhea (Poole et al., 2019).
Cancer Pain Management
Pain is one of the most common adverse effects of cancer and one of the biggest negative effects on a patient’s quality of life. Multiple studies and clinical practice have shown that premedicating before known painful procedures, medicating prophylactically for anticipated pain, and medicating for pain before it becomes too severe are proven to be the most successful means of managing pain. There are many pharmacologic and nonpharmacologic means of preventing and alleviating lung cancer pain.
Various subclassifications of analgesics can be used to prevent or treat the pain from lung cancer, including acetaminophen (Tylenol), aspirin, and ibuprophen (Advil or Motrin). (Aspirin and ibuprofen are also classified as non-narcotic anti-inflammatory drugs [NSAIDs].) Antidepressants such as duloxetine (Cymbalta) or anticonvulsants such as gabapentin (Gralise or Neurontin) are also used effectively against cancer pain in some situations.
Routes of administration are primarily oral, but acetaminophen can also be given rectally or by intravenous drip. Acetaminophen has no significant side effects, but long-term usage can cause hepatic disease. It is recommended that the daily dosage of acetaminophen not exceed 3,000 mg. NSAIDs may have side effects of dyspepsia. Gabapentin side effects are dizziness, staggered gait, nystagmus, drowsiness, and xerostomia (dry mouth).
Opioids are the strongest medications available to treat pain. There are a variety of medications that may be ordered for the extreme pain that results from cancer, such as morphine, oxycodone, fentanyl, and methadone. The routes of administration are oral, topical, intramuscular, and intravenously.
Opioids carry with them the possible stigma of addiction or a loss of control due to dependency on the drugs for pain relief. Older adults in particular may be fearful that the use of opioids will cause them to become addicted. This may cause patients who are in extreme pain to forego taking sufficient prescribed medications. It is essential that patients take sufficient opioids to preserve a successful quality of life. The patient and family must also be educated that the stress caused by chronic pain may interfere with the patient’s ability to heal.
The most common side effects of opioids are meiosis, constipation, itching, respiratory depression, hypotension, and euphoria. The side effects are more common with the injectable routes.
Cannabinoids (medical marijuana) are a more recent and remarkably effective classification of drugs used to treat all forms of cancer. Cannabinoids contain THC, which is the active ingredient found in marijuana. The majority of U.S. states have legalized medicinal marijuana for treating chronic pain and the nausea and vomiting that often accompanies cancer treatments.
Several research studies involving individuals undergoing cancer treatment have shown that medical marijuana can work similarly to opioids when treating patients living with chronic pain. They also have anti-inflammatory effects that can effectively treat cancer-related pain. Cannabinoids can be given in conjunction with opioids for better effect. The routes of administration for cannabinoids are smoking, vaporizing, oral, and subcutaneous spray. Common side effects of cannabinoids are lethargy, dizziness, dry mouth, increased appetite, and paranoid ideation.
Other modalities that have proved effective in treating all forms of pain, including cancer pain, include physical therapy, acupuncture, acupressure, yoga, diversion, guided imagery, and massage. Radiation treatment used to shrink the size of a lung tumor can also reduce pain (LungCancer.org, 2020).
REHABILITATION THERAPY
Rehabilitation therapy in lung cancer treatment can help patients maintain and restore physical and emotional well-being more quickly and more fully. A self-management rehabilitation program is structured to motivate and support patients to adopt positive health practices and to develop skills to better manage their illness.
Pulmonary Rehabilitation
Pulmonary rehabilitation (PR) is a comprehensive, evidence-based, multidisciplinary program designed to assist patients with lung cancer who are having difficulty with breathing and activities of daily living (ADLs). The primary objective of a PR program is to restore individual patients to as independent a level of function as possible with an improved health-related quality of life.
Most PR programs involve a respiratory therapist, occupational therapist, physical therapist, and dietitian. Physicians, pharmacists, and nurses may also be involved. PR programs are delivered in inpatient, outpatient, clinic, physician office, telehealth, and home settings. PR programs include assessment, exercise therapy, education, and psychological support. Some PR programs continue for an extended time, but most run for a few weeks and then provide patients individualized instructions for continuing at home.
The benefits of PR include maximizing the patient’s functional status and reducing healthcare costs by promoting self-management of symptoms. Evidence has shown that dyspnea symptoms improve in patients with lung cancer who undergo a PR regime. PR is also proven to be a cost-effective treatment model that reduces the number of hospital readmissions, although it cannot be substantiated that PR extends the life of patients with lung cancer (Harding et al., 2020).
PROGRAM PLANNING
PR programs are tailored to the needs of the individual patient. The number of sessions per week, need for nutritional counseling, need for psychological support, and need for oxygen supplementation are determined after a careful assessment of the patient’s capabilities and desired goals. The best possible benefits occur in a PR program lasting six to eight weeks. There is no evidence of any additional benefits from expanding the program to 12 weeks’ duration (GOLD, 2019).
PATIENT AND FAMILY EDUCATION
Patient and family education are central to all PR programs. In these education sessions, patients and their families learn details about lung cancer and its treatment. Education informs the patient and family in how to self-manage the disease in collaboration with the various PR disciplines. Education topics may include understanding chronic lung disease, medications, breathing control, oxygen therapy, heart health, falls prevention, diagnostic tests, and advance care planning. Increasing patient knowledge leads to essential behavior changes, although education alone does not improve outcomes (GOLD, 2019).
EXERCISE TRAINING
Pulmonary rehabilitation seeks to optimize the functional status of a patient with lung cancer via supervised, progressive exercise training and collaborative and supported self-management. Patients diagnosed with lung cancer are usually—though not always—older adults with both extensive smoking history and poor cardiopulmonary fitness. Physical inactivity related to lung cancer can lead to exercise intolerance, skeletal and respiratory muscle wasting, and decreased functional endurance.
Appropriately selected and supervised exercise is considered safe and valuable for most patients with cancer regardless of age or stage of life. While exercise does not improve lung function, it can reduce symptoms and increase exercise tolerance. Exercise has been shown to improve muscle strength/tone, range of motion (ROM), endurance, and lung capacity, and it may reduce hospitalizations for acute exacerbations. Exercise may also promote weight loss and anxiety reduction, as well as improve heart rate and blood pressure (Zeng et al., 2018; Garcia et al., 2016).
Exercise training in a pulmonary rehabilitation setting may be supervised by physical therapists, occupational therapists, exercise physiologists, advanced practice nurses, or other appropriately trained clinicians. Exercise type, frequency, and duration are individualized to patient needs, with clinicians monitoring patient status during exercises, determining when patient regimens should be advanced, and assessing patient progress. Typically, PR programs include multiple components, as described below:
- Aerobic exercises, such as walking or stationary bicycling (usually 3x/weekly). A regimen might begin with slow treadmill walking for a few minutes, with gradual increases as tolerated.
- Strength training, utilizing either free weights or machines to provide resistive exercise. Initially, a repetition range of 8–12 may be appropriate, with incremental increases added when the current workload can be performed for one to two repetitions. Recommended training frequency is approximately 2–3 days/week (Zeng et al., 2018).
- Endurance training may reduce lung hyperinflation, exertional dyspnea, and muscle dysfunction in patients with lung cancer, while promoting heart rate recovery. Endurance training, such as walking, cycling, and upper extremity exercises (if tolerated), may slow the progression of activity intolerance in patients with lung cancer (Zeng et al., 2018).
- Supplemental oxygen may be indicated during exercise for patients who experience severe exercise-induced hypoxemia. Oxygen saturations should be monitored in patients with supplemental oxygen dependency. Increased flow rates may enable oxygen-dependent patients to exercise longer and with less dyspnea.
- Ongoing support and encouragement are important components of any patient care regimen, including PR. Counseling and periodic check-ins (via e-mail, phone, or text) may help patients stay motivated and feel supported. When a functional plateau is reached in the clinical setting, patients may be assigned a home-based maintenance program (including activities such as stretching, weight training, or stationary cycling).
Occupational Therapy (OT)
Occupational therapy is an important aspect of rehabilitation following lung cancer treatments and improves quality of life. OT focuses on symptom management, reduction of fatigue, respite from shortness of breath, activities of daily living, pain management, mental health, quality of life, return to work, and resumption of community participation, as applicable. OT can be conducted in a variety of venues along the healthcare continuum, including general or specialty hospitals, rehabilitation centers, hospice units, and the home.
There is strong evidence that exercise plans set up and supervised by OTs reduce cancer-related fatigue and improve the quality of life for the patient (see “Exercise Training” above). Interventions such as problem-solving and energy conservation can reduce breathlessness and allow patients to better tolerate increases in exercise and activities. Pain management is another category in which OTs work with patients via improved sleep and exercises to further improve their quality of life (Hunter et al., 2017a; White, 2016).
ASSESSMENT AND PLANNING
OTs begin with a detailed assessment and history-taking of the patient in order to develop an individualized plan of care. Established assessment tools and tests include:
- Model of Human Occupation Screening Tool (MOHOST), to identify and measure possible occupational dysfunction, including motor skills, employment, and communication
- Canadian Occupation Performance Measure (COPM), a standardized occupational profile to focus the patient on self-measurement of occupational progress
- Brief Fatigue Inventory (BFI), to measure cancer-related fatigue that may be related to pain
- Rivermead Behavioral Memory Test (RBMT), to evaluate everyday memory tasks such as names of objects, facial recognition, and recall
- Activity Measure for Postacute Care (AM-PAC), a quick assessment to measure postacute discharge outcomes
- Worker Role Interview (WRI), to explore psychosocial and environmental concerns regarding returning to work
(Braveman et al., 2017)
ACTIVITIES OF DAILY LIVING
The need for assistance with activities of daily living (ADLs) (bathing, eating, ambulation, dressing, and grooming) may be partial or complete as well as temporary, rehabilitative, or permanent. Certain reductions in capabilities due to confusion, fatigue, weakness, or difficulty in transferring can affect the patient’s ability to perform ADLs effectively.
Patient preferences are paramount in the use of rehabilitation to improve their execution of ADLs. Patient involvement in planning ADL interventions provides buy-in and often encourages the patient to work harder for success.
Depending on the circumstances, the family and/or caregiver(s) may also need to be educated and trained to assist the patient with ADLs. A well-trained family or caregiver may be more successful than healthcare staff because of their familiarity with the patient. Availability of such caregivers may allow the patient to be discharged earlier. Having the family assist with ADLs may also be a cultural consideration.
INSTRUMENTAL ACTIVITIES OF DAILY LIVING
Occupational therapy may also address instrumental activities of daily living (IADLs) (i.e., tasks that require more complex cognitive abilities such as shopping, check-writing, cooking, housecleaning, doing laundry, taking medications, accomplishing transportation, using communication devices, home maintenance, etc.).
OTs play a vital role in helping a patient with lung cancer return to work, perform housekeeping duties, manage medications, and more. For instance, high-intensity exercise that includes strength training, interval training, and a home-based exercise program after discharge from the hospital may help to minimize any decrease in the patient’s abilities. Depending on the type of work the patient does, the OT will design exercises, suggest adaptive devices, and promote increased endurance so that the patient is able to return to work (Hunter et al., 2017b). (See also “Exercise Training” above.)
ENERGY CONSERVATION
Energy conservation assists patients in minimizing muscle fatigue, pain, and stress on involved joints. Moderating activities and ensuring a sufficient amount of rest also help patients to maintain function. Setting up the environment to make carrying out ADLs and work-related performance extends the patient’s ability to function and maximizes comfort. These changes promote independence and allow the patient to continue to be able to accomplish their desired goals throughout each day and until the end of the day (Duke University, 2020).
ENERGY CONSERVATION TIPS
- Simplify tasks and set realistic goals; it is not necessary to do things the same way they have always been done.
- Plan activities (chores, exercise, and recreation) ahead of time. Space out activities throughout the day. Do not schedule too many things to do in one day. Do the things that take more energy when feeling better.
- If needed, rest before and after activities.
- When becoming tired during an activity, stop and rest; it may be necessary to finish activities on another day or when feeling less tired.
- Do not plan activities right after a meal; rest 20 to 30 minutes after each meal.
- Ask for help; divide tasks among family and friends.
- Get a good night’s sleep and elevate one’s head when sleeping. Be careful not to nap too much during the day, since this may cause difficultly sleeping at night.
- Carry out grooming activities (shaving, drying hair, etc.) while sitting.
- If needed, use assistive devices and tools such as a walker, shower chair, hand-held shower head, bedside commode, or long-handled tools for dressing (such as a dressing stick, shoehorn, or sock donner).
- Wear clothes that have zippers and buttons in the front to avoid having to reach behind oneself.
- When climbing steps, rest part of the way if tired. Arrange activities in order to avoid climbing up and down stairs many times during the day.
- Avoid extreme physical activity. Do not push, pull, or lift heavy objects (more than 10 pounds) that require any strain.
(Cleveland Clinic Foundation, 2018)
HEALTH PROMOTION
A health promotion program can help patients maximize their ability to perform the activities that are meaningful, enjoyable, and necessary to them. The best health promotion program serves to prevent dysfunction; promote and enact a healthy lifestyle; and facilitate recovery from injury, disease, or developmental deficits. Health promotion can improve physiologic and psychological concerns related to cancer and prolong life. Elements of health promotion programs as taught by occupational therapists include skills training in socialization, caregiving, parenting, time management, activities organization, and stress management (ALA, 2021).
SLEEP THERAPY
Sleep deprivation is a common problem for patients with cancer and may be related to stress, pain, and the physical effects of the disease and its treatments. Lack of sleep is a possible cause of automobile accidents, workplace accidents, increased healthcare visits, poor work productivity, and diminished quality of life. Occupational therapists assess and address the implications of sleep insufficiency in patients (AOTA, 2021).
Assessing the sufficiency of patient’s sleep involves evaluating difficulties in sleep preparation; sleep participation; and how long it takes to fall asleep, sleep duration, the ability to stay asleep, or daytime sleepiness.
Work, shift work, school, caregiving responsibilities, pain, and excessive fatigue may affect the ability to sleep well. Disturbances in balance, vision, strength, skin integrity, and sensory systems are also contributing factors. Psycho-emotional status, including depression, anxiety, and stress, may prevent patients from falling and staying asleep. Substances such as caffeine, nicotine, drugs or alcohol, smoking, or certain medications may need to be withheld to improve sleeping patterns (AOTA, 2021).
Occupational therapists use the following interventions to help patients prevent sleep deprivation:
- Educating clients and caregivers on sleep misconceptions and expectations
- Addressing secondary conditions that may precipitate diminished sleep quality (e.g., pain, decreased range of motion, depression, anxiety)
- Encouraging health management behaviors such as smoking cessation, reduced caffeine intake, a balanced diet, and adequate exercise
- Establishing predictable routines, including regular times for waking and sleeping
- Managing pain and fatigue
- Addressing performance deficits or barriers to activities of daily living, particularly for bed mobility and toileting
- Establishing individualized sleep hygiene routines (e.g., habits and patterns to facilitate restorative sleep)
- Teaching cognitive-behavioral and cognitive restructuring techniques, such as leaving the bedroom if awake and returning only when sleepy, or exploring self-talk statements regarding sleep patterns
- Increasing coping skills, stress management, and time management
- Addressing sensory disorders and teaching self-management or caregiver management
- Modifying the environment, including noise, light, temperature, bedding, and technology use while in bed
- Advocating on a state or national level for laws that protect workers from excessive work schedules that threaten their health or public safety
(AOTA, 2021)
Smoking Cessation
Smoking cessation is the most important measure a patient can take preoperatively to prepare for lung cancer surgery and prevent complications postoperatively. Since the vast majority of patients with lung cancer have some history or current use of cigarettes, smoking cessation is prioritized, particularly if the patient is to undergo surgical resection. Coughing and secretion production will improve and be much less of a problem postoperatively.
Counseling and medications are effective in assisting a patient with smoking cessation. Information about telephone helplines and brochures can be given to the patient and family or caregiver(s) to support stopping smoking. Non-nicotine medication such as oral varenicline (Chantix) acts as an agonist at the nicotine receptors to ease the withdrawal symptoms. The antidepressant buproprion (Zyban) causes a reduction in the urge to smoke and reduces some withdrawal symptoms. Nicotine replacement medications may be used, such as Nicorette gum, Nicotrol nasal spray, or Habitrol or NicoDerm skin patches. Organized cessation programs can be effective. These include hypnosis, acupuncture, behavioral interventions, aversion therapy, group and individual therapy, and self-help opportunities.
Most smokers resume smoking within three months of quitting. Therefore, it is important to teach ex-smokers to identify their own triggers that might cause them to resume smoking. These could be drinking alcohol (which lowers inhibition), being around other smokers, having cigarettes or other smoking devices easily available, stress, or depression (Harding et al., 2020).
CASE
Erlene has active lung cancer. She has been experiencing fatigue and weakness as side effects of her treatment and recently suffered a fall. Her nurse practitioner, Caitlyn, referred Erlene to physical therapy and occupational therapy, where she has received training in functional mobility, safety and falls prevention, therapeutic exercise, energy conservation, and ADLs.
At her latest visit with her NP, Erlene describes a cough and shortness of breath and states, “I’ve been smoking all of my life and I’ve tried to quit before on my own. But I guess it’s time I try again.” Caitlyn asks her if she would be interested in learning about some ways that can help in quitting smoking, and Erlene agrees. Caitlyn discusses with her possible triggers to smoking, such as drinking, smoking after each meal, stress, and possible depression. She also recommends a prescription for pills to help Erlene stop smoking. She explains to Erlene that many smokers restart within three months after quitting and gives her information about smokers’ help lines and local 12-step Smokers Anonymous groups.
Caitlin documents her discussion with Erlene so that all of the necessary members of her healthcare team can stay informed of what steps have been taken regarding smoking cessation in order to help Erlene achieve her goals.
Nutritional Therapy
A patient with lung cancer tends toward submaximal weight and body size for many reasons. The disease itself can cause a loss of appetite based on symptomatic ill-feelings. Malignant cells and the body’s other cells are in competition for nutrients. Cancer treatments such as chemo- and radiation therapy may cause anorexia, nausea and vomiting, and altered taste. These all interfere with the patient’s ability to take in a sufficient amount of nutrients. Patients with malnutrition have an increase in morbidity and mortality.
A stable nutritional status promotes healing and improves a patient’s quality of life. A registered dietitian (RD) can provide dietary recommendations based on the patient’s food preferences, other dietary needs, and individual caloric requirements. These recommendations are shared with any caregivers who may be responsible for shopping and cooking for the patient.
Systemic therapies are more likely than other treatments to cause nausea and vomiting, depending on the particular medication and dose. Higher doses are not tolerated as well. The symptoms can start immediately or up to 24 hours after the dosage has been completed. Radiation therapy affects the epithelial cells lining the GI tract, causing GI symptoms such as anorexia, diarrhea, intestinal stricture, xerostomia, and pain. Targeted therapies and immunotherapy also have nausea and vomiting as an adverse effect.
Therefore, a patient with lung cancer who is undergoing these therapies requires special attention to maximize their intake of necessary fluids and nutrients. A special protein and calorie-rich diet can be set up for the patient, prioritizing their food and drink preferences to encourage them to eat and drink more. Chilled foods and drinks are often more palatable, as are frequent, small portions and snacks (ACS, 2020c; Potter et al., 2019).
The patient’s participation in food-related activities such as shopping, cooking, and meal preparation can also minimize the risk of disease progression and resultant disability. The federal Supplemental Nutrition Assistance Program (SNAP) is one source of financial aid available to ensure the patient has access to an adequate amount of healthy food. The patient may need additional assistance beyond financial support, such as help with transporting, preparing, and cooking food, to promote optimal nutritional intake. These needs are often addressed by the occupational therapist (Juckett & Robinson, 2019).
Integrative Oncology (IO)
Integrative oncology is a holistic therapy that combines traditional medical cancer treatments such as surgery, systemic therapies, and radiation therapy with alternative therapies such as yoga, meditation, music therapy, support groups, journaling, tai chi, and crafts. Alternative therapies are often effective for preventing and treating nausea, joint and other pains, lack of sleep, and loss of appetite. These therapies can also relieve stress in patients and their families. Nutritional therapy (see above) is an important part of integrated oncology and is included in the patient and family education (Ironwood Cancer & Research Center, 2020).
ACUPUNCTURE
Acupuncture is a traditional Chinese therapy that extends back thousands of years. Multiple thin, sterilized, solid needles the diameter of a hair are carefully inserted into points along the body’s meridians (energy channels) to stimulate healing energy known as qi or chi, according to Chinese medical theory. Acupuncture sites can also be stimulated by heat (moxibustion), acupressure, friction, suction (cupping), and electromagnetic energy impulses. Accessing this flow of energy is believed to make acupuncture treatments effective.
However, an invasive procedure such as acupuncture may be contraindicated where there is an active cancer.
While some consider the practice to be controversial, National Institute of Health (NIH) studies have demonstrated that acupuncture is evidence-based and effective in treating a wide range of conditions:
- Nausea caused by surgical anesthesia and systemic therapies
- Dental pain after surgery
- Addiction
- Headaches
- Menstrual cramps
- Tennis elbow
- Fibromyalgia
- Myofascial pain
- Osteoarthritis
- Low back pain
- Carpal tunnel syndrome
- Asthma
(JHM, 2020b)
MASSAGE
Massage is deep or superficial pressure over skin, muscles, tendons, and ligaments for relaxation of the body parts. Examples of massage therapy include Swedish, deep tissue, sports, and trigger point. Massage has been found to be effective for:
- Anxiety
- Digestive disorders
- Fibromyalgia
- Headaches
- Insomnia related to stress
- Myofascial pain syndrome
- Soft tissue strains or injuries
- Sports injuries
- Temporomandibular joint pain
(Mayo Clinic, 2020c)
It is possible for massage to cause deleterious effects on patients with deep vein thrombosis (DVT) because of the possibility of dislodging the clot, patients with bleeding disorders, patients on anti-coagulation therapy because of an injury causing bleeding, and patience with burns, skin wounds, severe osteoporosis, bone fractures, and thrombocytopenia.
YOGA
Yoga is recognized for its ability to promote physical and mental well-being. Different aspects of yoga include physical postures (asanas), breathing techniques (pranayama), and meditation. Yoga performed by patients has the benefit of contributing to mental health, quality of life, improved sleep, and a greater sense of well-being. It also contributes to deep relaxation and decreases stress (Hunter et al., 2017a).
Yoga can be used to:
- Treat low-back pain and neck pain
- Relieve menopause symptoms
- Manage anxiety or depressive symptoms associated with difficult life situations (but yoga has not been shown to help manage anxiety disorders, clinical depression, or posttraumatic stress disorder [PTSD])
- Help people quit smoking
- Help people who are overweight or obese to lose weight
- Improve quality of life
(NIH, 2020a)
GUIDED IMAGERY
Guided imagery is a concentrated type of relaxation that balances the mind and body. In this practice, the imagination is used to create calm, peaceful images in order to provide an escape from worries or stress. Guided imagery may:
- Enhance coping skills
- Lower and regulate heart rate
- Lower blood pressure
- Lower and improve respirations
- Provide inner strength, hope, and courage
- Promote relaxation
- Help control pain
(Cleveland Clinic Foundation, 2020)
COMPLICATIONS OF LUNG CANCER
There are several complications secondary to lung cancer. One of the most feared complications is a recurrence of the original disease. This may be in the same site, in nearby lymph nodes, or in a different body system due to metastasis. Pneumonia is another common complication, usually caused by neutropenia from systemic therapies or smoking, that greatly increases mortality rates in the patient with lung cancer.
Lung cancer may also cause the abnormal secretion of hormones, enzymes, or cytokines, resulting in neoplastic syndromes. Endocrine syndromes include abnormal secretion of antidiuretic hormone or vasopressin, polycythemia, and Cushing’s syndrome. Rare neurological disorders caused by lung cancer include Eaton-Lambert reverse myasthenic syndrome, subacute cerebellar degeneration, and limbic encephalopathy. Hypercalcemia can be caused by the secretion of an excess of a parathyroid hormone-like substance from a malignant tumor.
Recurrence
Lung cancer recurrence can be defined by where it occurs:
- Local recurrence is when cancer returns in the lung near the site of the original tumor.
- Regional recurrence is when cancer recurs in the lymph nodes near the site of the original tumor.
- Distant recurrence is when lung cancer recurs far away from the original tumor, such as in the bones, brain, adrenal glands, or liver.
A patient with lung cancer must have a period of remission of greater than 3 months in order for recurrence to be considered more than disease progression. The most common form of the disease, NSCLC, has a recurrence rate of 30%–55%. Recurrence depends primarily on the stage of the lung cancer at the time of diagnosis. Stage I has a recurrence rate of approximately 30% within 5 years, while stage IV recurs in up to 70% patients within 2 years.
With SCLC, which is not as treatable as NSCLC, the recurrence rate can be up to 70% within 1–2 years of treatment. The rate of recurrence after 5 years is rare.
The most common cause of lung cancer recurrence is the spread of the original cancer. Prevention of recurrence is largely based on the success of changing modifiable risk factors such as smoking (Verywell Health, 2021).
FEAR OF RECURRENCE
Fear of recurrence is a common characteristic of patients with cancer across all diagnoses, genders, races, and ages. Even when the treatment is successful, patients may be afraid that their cancer will return either in the primary site or as a metastasis. In a study using a seven-step fear of recurring cancer scale (FRCS) to measure participants’ level of fear of recurrence, 66.2% experienced a clinical level of fear of recurrence. Higher scores on the scale were unrelated to age, performance status, or quality of life (Rha et al., 2022).
Metastasis
Lung cancer most commonly spreads to the lymph nodes either in the lung tissue or in the airways. When there is no further spread beyond the lungs, it is not considered metastatic. If there is further metastasis, it usually results in hard lumps in the neck or the axillae where there are lymph nodes.
However, metastasis is a condition frequently related to lung cancer. Since lung cancer is slow growing, it is not unusual for a metastatic tumor to be diagnosed at the same time the lung cancer itself is diagnosed. The presence of metastasis causes a poorer prognosis, with a shorter life expectancy regardless of the kind(s) of treatment given.
Some metastatic tumors are slow growing, such as lung cancer with metastasis to the breast, pancreas, and thyroid. It is not unusual for the lungs and thyroid glands to be concurrent primary sites, resulting in a poor life expectancy.
BRAIN METASTASIS
Lung cancer is the most common primary cancer site for brain metastasis. Up to 40% of all lung cancers result in metastasis to the brain. This is exacerbated by the presence of nicotine, including in the form of a nicotine patch or gum. The nicotine skews the polarity of M2 microglia, macrophages that remove infections and damaged neurons from the central nervous system (Wu et al., 2020).
Often, lung cancer metastasis to the brain occurs so quickly that the brain metastasis is diagnosed well before the primary lung cancer is recognized. This can often be prevented by treatment with prophylactic cranial irradiation (PCI).
SYMPTOMS OF METASTASIS TO THE BRAIN
- Headaches
- Nausea and vomiting
- Seizures
- Loss of balance and coordination
- Difficulty with speaking
- Vision changes
- Weakness on one side of the body
- Fatigue
(Eldridge, 2020)
Diagnosis is made by a computerized tomography (CT) scan of the brain. Treatment may be corticosteroids to treat or prevent swelling and symptomatic medications such as analgesics, anti-emetics, and anticonvulsants. Radiation therapy focused on the brain tumor may be given to reduce symptoms from the tumor. When the brain metastases are few, they are referred to as oligometastasis and may be treated with either surgery or stereotactic body radiotherapy (SBRT), cyber knife, or gamma knife.
BONE METASTASIS
Lung cancer frequently metastasizes to the bones. As many as 30%–40% of patients with lung cancer experience metastasis to the bones. The most common site of this metastasis is the spine and vertebrae. The pelvis is also an area of metastasis that is hard to treat and has a 40% rate of recurrence. Lung cancer may metastasize to the femur, humerus, hands, and feet as well (Eldridge, 2020).
Bone metastasis causes the development of bone pain, fractures, hypercalcemia, and nerve compression. With bone cancer, osteoblasts secrete cytokines that cause bone reabsorption, leading to symptoms suggesting the need for diagnostic testing to rule out the cause. Patients whose cancer metastasizes to the bone have an extremely poor prognosis (Teng et al., 2020).
LIVER METASTASIS
Liver cancer is one of the most common metastases secondary to lung cancer. This is usually an asymptomatic cancer, but in the later stages the following symptoms may appear:
- Loss of appetite
- Weight loss
- Fatigue
- Bloating and leg swelling (edema)
- Itching
- Jaundice, yellowing of the skin or whites of the eyes
(Eldridge, 2020)
When lung cancer metastasizes to the liver, there are usually several other organ sites of metastasis and the prognosis is usually poor, with a short life expectancy. Multiple metastatic sites will preclude the possibility of corrective surgery due to this poor prognosis. When the liver is the only site of metastasis, surgery is more likely to be performed. It is not uncommon for the liver cancer to exhibit symptoms after the lung has already been resected surgically; hepatic surgery may be successful in extending life expectancy in this case (Hokada et al., 2019).
Liver metastasis is diagnosed with an abdominal ultrasound, a CT scan, or a positive emission tomography (PET) scan. Systemic therapies are usually employed to treat the liver and the primary lung tumor (Eldridge, 2020).
STOMACH METASTASIS
Small cell lung cancer is frequently accompanied by a distant metastasis, such as to the stomach. The symptoms of stomach cancer in the presence of other cancers are not definitive, and the diagnosis of metastasis to the stomach is often made upon an autopsy examination. SCLC has a rapid progression and a poor prognosis. Systemic therapies used to treat SCLC can also be beneficial in treating metastatic stomach cancer (Peng et al., 2019).
ADRENAL GLANDS METASTASIS
Lung cancer may metastasize to the adrenal glands. Adrenal metastasis is usually asymptomatic and discovered when a scan is done to stage the primary tumor. Excision of the tumor is usually successful.
PANCREAS METASTASIS
Cancer of the pancreas rarely occurs as a form of metastasis secondary to lung cancer. The identifying symptoms are pancreatitis, obstructive jaundice, and lumbar back pain, found in 43% of cases. The other 57% of cases are asymptomatic for pancreatic symptoms. The average life expectancy for pancreatic cancer due to metastasis from lung cancer is 8.8 months. Patients with pancreatic symptoms have a shorter life expectancy than those who are asymptomatic. Systemic therapies significantly prolong life in the case of pancreatic cancer. Radiation therapy to the pancreas helps to relieve symptoms but does not extend life (Zhang, 2020).
Paraneoplastic Syndromes
The tumor cells of a person with lung cancer or the antibodies produced by the tumor cause the secretion of hormones, enzymes, and cytokines that destroy healthy cells. These substances can cause one or more paraneoplastic syndromes. Paraneoplastic syndromes are rare disorders that may be caused by a compromised immune system responding to a tumor or production of a hormone, enzyme, or cytokine.
Symptoms may include fever, night sweats, weight loss, and decreased appetite and can affect many organ systems (neuro, skin, endocrine, hematologic systems). The ectopic secretion of these substances can cause a metabolic emergency that may be evident before the symptoms of the lung cancer are evident. These syndromes may show improvement when the underlying tumor is treated and either reduced in size or removed (Harding et al., 2020; Tan, 2019).
ENDOCRINE SYNDROMES
Syndrome of Inappropriate Antidiuretic Hormone (SIADH)
A cancerous tumor in the lung can cause the abnormal secretion of antidiuretic hormone or vasopressin. The results are a concentrated urine and diluted plasma. The symptoms of SIADH and hyponatremia are the same. They mostly include central nervous system problems and are exhibited when the plasma osmolality falls to <240 mOsm/kg. Symptoms include changes in mental status, altered personality, lethargy, and confusion. As the serum sodium falls to <115 mEq/L, symptoms progress to stupor, neuromuscular hyperexcitability, hyperreflexia, seizures, coma, and eventually death if the condition goes untreated. Treatment is with water restriction, sometimes with oral salt pills or intravenous 3% sodium chloride (Harding et al., 2020).
Polycythemia
Polycythemia vera is a condition in which the overproduction of red blood cells causes the blood to become hyperviscous and hypervolemic. A malignant tumor in the lungs can artificially induce hypoxia, causing the kidneys to hypersecrete erythropoietin to stimulate the bone marrow to produce new red blood cells. The symptoms of polycythemia are secondary to the hypertension caused by the hypervolemia and the hyperviscosity of the blood. They include headache, vertigo, dizziness, tinnitus, visual disturbances, pruritus, and paresthesias. Worsened symptoms may exhibit as angina, heart failure, intermittent claudication, and thrombophlebitis (Harding et al., 2020).
Cushing Syndrome
Cushing syndrome is a disease in which the body is saturated with corticosteroids in the form of glucocorticoids. Ectopic adrenocorticotropic hormone (ACTH) production can be caused by several types of tumors, including lung cancer. Clinical manifestations of Cushing syndrome are multisystem and a result of the body’s response to excessive corticosteroid production. Weight gain, redistribution of adipose tissue, hyperglycemia, muscle wasting, osteoporosis, loss of collagen, and reddish striae on the torso are the most common symptoms (Harding et al., 2020).
NEUROLOGIC SYNDROMES
Eaton-Lambert Reverse Myasthenic Syndrome
Eaton-Lambert reverse myasthenic syndrome is a rare neurologic condition that interferes with the messages normally sent from the nerves to the muscles, impacting the muscle’s ability to contract. Middle-aged or older adults with lung cancer comprise approximately 50% of all Eaton-Lambert reverse myasthenic syndrome cases. The symptoms appear gradually over weeks to months, including weakness in the legs, arms, neck, and face, and difficulty with controlling automatic body functions such as blood pressure. Other symptoms that occur fairly commonly are muscle pain, difficulty walking, difficulty with stairclimbing, inability to lift or raise the arms, drooping eyelids, dry eyes and mouth, visual blurriness, dysphagia, orthostatic dizziness, constipation, and erectile dysfunction (NHS, 2019).
Paraneoplastic Cerebellar Degeneration
A paraneoplastic cerebellar degeneration (PCD) is a rare complication of a tumor. PCD causes the production of antibodies that attempt to work against the tumor-produced antigens (which are a type of protein). The cancer-fighting antibodies may inadvertently attack these normal protein cells in the cerebellum. Clinical manifestations may include mild dizziness, nausea, vertigo, and nystagmus that may suggest a peripheral vestibular problem. These symptoms precede ataxia of the limbs, oscillopsia, dysarthria, tremor, and sometimes dysphagia and blurred vision (Tan, 2019).
Subacute Sensory Neuropathy
Subacute sensory neuropathy is an inflammatory disorder of the central nervous system. Although various malignancies can cause subacute sensory neuropathy, 80% of cases are caused by small cell lung carcinoma in the bronchi. There is no effective treatment for this disorder, but the use of immunosuppressants may help to improve the symptoms (Tan, 2019).
Limbic Encephalopathy
SCLC can attack the proteins that are necessary for the development, maturation, and maintenance of the vertebrate peripheral nervous system. The symptoms of limbic encephalopathy may include memory loss, personality changes, anxiety, depression, neuropsychiatric disturbances, partial or generalized seizures, status epilepticus, sensory hallucinations of smell and taste, and sleep disturbances.
HYPERCALCEMIA
Hypercalcemia can be caused by an excess of a parathyroid hormone-like substance secreted by malignant tumors such as lung cancer. Natural parathyroid hormone (parathormone) works to produce a therapeutic amount of calcium in normal circumstances. A calcium level greater than 12 mg/dl will most likely cause adverse symptoms. The most common symptoms are apathy, depression, fatigue, muscle weakness, ECG changes, polyuria, nocturia, anorexia, and nausea and vomiting (Harding et al., 2020).
COVID-19
Research studies have shown that patients with lung cancer are at a significantly increased risk of contracting the COVID-19 virus. Radiation and systemic therapies for cancer affect the immune system, contributing to the higher risk (Passaro et al., 2021).
A prospective observational study of 800 patients from 55 hospitals showed the mortality rate of COVID-19–positive patients with cancer was not different from COVID-19 patients without cancer. The risk factors for higher mortality rates in both groups proved to be age >60 years, male sex, hypertension, cardiovascular disease, COPD, and diabetes. The results of the study highlighted these factors as a more important consideration than COVID-19 positivity when deciding about systemic cancer treatments (Haider et al., 2021).
PALLIATIVE CARE
The purpose of palliative care is to provide care for patients with life-limiting illnesses. The focus of care for the patient at this time is to keep them comfortable. This type of care can be started at the beginning of the patient’s diagnosis (as opposed to hospice care, which is initiated when all acute care and curative treatments have been ceased).
The goals of palliative care are to:
- Provide relief from symptoms, including pain
- Affirm life and neither hasten nor postpone death
- Support holistic patient care and enhance quality of life
- Offer support to patients to live as actively as possible
- Offer support to the family during the patient’s illness
(Harding et al., 2020)
Common palliative treatments for patients with lung cancer include addressing:
- Nausea during systemic therapy treatments
- Postoperative or any other kind of pain
- Depression throughout the course of the illness
- Constipation from narcotic pain medications
(Hospice Alliance, 2020)
Palliative Pharmacology Treatment
The purpose of palliative care is to foster comfort and as much independence as possible when a patient receives a life-limiting diagnosis and through the course of their care. When there is a question whether a patient in palliative care needs medications or not, the medications are given.
- Pain is the most frequent symptom in terminal illnesses. The most common classification of analgesics for this patient population is narcotics. Narcotics are highly effective for treating severe pain but can cause several side effects such as respiratory depression, hypotension, itching, constipation, and more. If the patient does not have an IV or is no longer able to swallow, morphine sulfate liquid drops may be given sublingually and are therapeutic.
- Nausea and vomiting (n/v) are common sequelae of chemo- and radiation therapies. The antiemetic used almost exclusively if there is IV access is ondansetron HCl (Zofran). An over-the-counter antiemetic that is effective for palliative care patients is dimenhydrinate (Dramamine). More recently, the cannabinoid dronabinol (Marinol) can be given in liquid form for easy swallowing or medical marijuana by inhalation to treat n/v and stimulate appetite.
- Delirium as a symptom of terminal patients can be treated by benzodiazepines and sedatives by injection or orally if the patient is able to swallow.
- Anxiety or restlessness is treated with an anxiolytic medication such as the benzodiazepine alprazolam (Xanax).
- Dyspnea due to hypoxia or a compromised airway can be treated with a bronchodilator or expectorant.
- Constipation is common and may be treated with a stool softener, laxative, suppository, or enema.
(Harding et al., 2020)
Palliative Physical Therapy
For a patient in palliative care, an important goal of physical therapy is to maximize the patient’s functional mobility independence. Special consideration is also given to the patient’s and family’s wishes with regard to their individual goals for physical therapy.
As with any patient, the therapist conducts an evaluation of the palliative care patient and determines the extent to which the patient can be physically active given postoperative pain, weakness following systemic therapies, and motivation in the face of a life-limiting diagnosis. In the patient with lung cancer, a palliative treatment plan may address management of breathlessness with breathing retraining, relaxation techniques, and the pacing of activities to ensure that the patient does not become too tired or excessively hypoxic. The plan of care, if the patient and family are in agreement, may also include assistance with mobilization abilities, practicing with gait aids such as walkers and canes, and exercises such as sit-to-stand practice (Physiopedia, 2022).
Palliative Occupational Therapy
Occupational therapists play an important role on palliative care teams by identifying life roles and activities that are meaningful to patients and addressing barriers to performing these activities. Occupational therapists working in palliative care address pain relief and symptom management, provide resources and education for patients and their families, and integrate psychological and spiritual aspects of care with the necessary medical treatments. Additionally, occupational therapists identify current and potential abilities and determine barriers to engaging in occupations, including activities of daily living, instrumental ADLs, rest and sleep, leisure, and social participation (AOTA, 2020).
Palliative Respiratory Therapy
The respiratory therapist is vital to the treatment of a patient with lung cancer throughout every aspect of their treatment and rehabilitation, including during palliative care. Oxygen and a clear airway are critical to the comfort of the patient. The RT conducts pulmonary assessments to determine and recommend the level of oxygen and treatments needed. At the end of life, when the patient goes through distinctive respiratory changes, the respiratory rate increases as the oxygen saturation decreases. The patient may experience Cheyne-Stokes breathing, with rapid respirations alternating with periods of apnea. There may be an inability to cough or independently clear their airway. Irregular breathing with a progressive slowing will occur until there is a cessation of respirations (Harding et al., 2020).
Surgical Procedures for Symptom Palliation
A stenotic airway can be splinted by tracheobronchial airway stents to relieve coughing, dyspnea, or respiratory insufficiency that may be caused by abnormal granulation tissue, lung cancer, metastatic cancers, infections, tuberculosis, lymphoma, or other inflammatory diseases. The stent is cylindrical and placed by bronchoscopy. An airway stent can prevent airway collapse and external compression, or can delay tumor growth advancing into the airway. The stent(s) can be permanent or removable. By maintaining adequate airway patency, atelectasis is prevented and the patient breathes easier (UCSDH, 2020).
For malignant tumors that remain inoperable, bronchoscopic laser interstitial thermal therapy can remove small, obstructive, bronchoscopic tumors. The process uses thermal energy that is transmitted to the cancerous tissue to remove by ablation via laser treatment. This method is safe and effective for the removal of small, peripheral endobronchial obstructions (Casal et al., 2018).
SURVIVORSHIP AND FOLLOW-UP CARE
Successfully surviving a diagnosis of lung cancer requires a program of follow-up that will enable the patient and their family to go forward whether the eventual outcome is a normal lifespan, recurrence of the cancer, metastasis, or early death. Providing information that is accurate but hopeful is one way healthcare personnel can help patients and their families make informed choices about how to proceed after curative treatment for lung cancer.
It is normal to experience fear and anxiety after receiving a diagnosis of lung cancer, even when the treatment has provided an encouraging outcome. Counseling or psychotherapy can help cancer survivors and their circle verbalize fears and concerns and learn mechanisms to deal with negative ideas. Focusing on wellness is a positive approach that allows patients to take part in their survival program with actions that will improve their health and participate in a multidisciplinary approach.
Nutrition, exercise, adequate sleep, awareness of symptoms, and risk reduction are areas where the patient can be given knowledge and support to deal with issues that can complicate postoperative or post-therapy progression. Registered dieticians, physical therapists, occupational therapists, sleep specialists, and counselors may be brought into the patient’s sphere if their primary care provider believes this will help to facilitate a better recovery period for the patient.
An essential part of survivorship is the management of long-term complications of lung cancer treatments. Symptoms that may ensue from these treatments are pain, infection, nausea and vomiting, skin burns, and nutritional deficits as a result of other side effects that are inadequately treated.
SURVIVORSHIP CARE PLANS
Survivorship care plans (SCPs) previously were mandatory for cancer survivors, but they are no longer required due to certain barriers in successfully implementing SCPs. SCPs were time consuming to prepare, there was no clarity regarding who should complete and maintain the information, and there was a lack of reimbursement for the completion and distribution of the SCP.
The current recommendation for SCPs is that the survivorship program team suggest available services and referrals and that usage of these be documented every year (Blaes et al., 2020).
Symptoms That Require Attention
Surveillance of patients with lung cancer in the postacute phase is essential for the survivor’s well-being, especially given the high rates of recurrence. It is recommended that the patient report the following symptoms to their primary caregiver, or surgeon if the patient is postoperative, to determine recurrence or metastasis:
| Body System | Symptoms |
|---|---|
| (Verywell Health, 2021) | |
| Local or in lymph nodes near the original tumor |
|
| Bones |
|
| Brain |
|
| Liver |
|
Routine Follow-Up Care
Patients should undergo surveillance imaging and a physical examination with their primary care giver frequently after the acute phase of lung cancer. Usually a CT scan is performed; this is to assess for recurrence or metastasis. The recommended frequency is every six months for the first two years, and then annually for detection of new primary lung cancers.
Brain magnetic resonance imaging (MRI) is not used for routine surveillance in stages I–III NSCLC but may be used every three months for the first year and every six months for the second year in patients with stages I–III SCLC who have undergone curative-intent treatment to assess for metastasis to the brain (Schneider, 2019).
Health and Wellness Recommendations
To promote a healthy lifestyle after active lung cancer, the American Lung Association makes the following recommendations for survivors of lung cancer:
- Stop smoking.
- Avoid secondhand smoke.
- Use psychological support services.
- Manage stress.
- Conserve energy to prevent fatigue.
- Eat nutritiously.
- Exercise.
- Develop and use a support system.
- Use helplines. (See “Resources” at the end of this course.)
CONCLUSION
Lung cancer is the most common cancer in the United States and globally. Smoking (cigarettes, cigars, e-cigarettes, or vaping) is the most common cause, with 80%–90% of all patients with lung cancer being current or previous smokers. The highest incidence of lung cancer in the United States is among men, with African American and White men presenting equally as the largest group.
Lung cancer is diagnosed by a thorough physical assessment and history-taking, accompanied by diagnostic tests, including a chest X-ray, CT scan of the chest, and ultimately a biopsy of lung tissue or fluid. There are two categories of lung cancer. Small cell lung cancer (SCLC) accounts for 20% of all lung cancers, is usually found nonresectable upon diagnosis, and generally has a terminal prognosis. Non-small cell lung cancer (NSCLC) accounts for the remaining 80% of lung cancers and, although fast growing, is largely resectable if there is not advanced staging.
The various types of lung surgery are a pneumonectomy, lobectomy, or partial or wedge resection. The decision that determines which surgical procedure to perform is based on tumor size, location, and patient performance status. Newer surgeries, including video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS), are minimally invasive and thus less painful, have shorter anesthesia times, and result in shorter hospital lengths of stay.
Other treatment modalities include chemotherapy, immunotherapy, and radiation therapy. There are many newer lung cancer chemotherapy and immunotherapy options that are proving effective with good outcomes, lower levels of recurrence, and fewer side effects. Newer forms of radiation therapy are also available that can target the tumor(s) more directly, leading to less destruction of the surrounding tissue. A complete lung cancer treatment plan will include one or all of these three main categories of treatments.
Other aspects of patient care include cancer rehabilitation, pulmonary rehabilitation, lifestyle interventions, including smoking cessation, promotion of complementary/alternative treatment methods, prevention measures, quality of life considerations, patient/family education, and palliative care. Ongoing clinical trials are being conducted to provide new evidence-based strategies to optimize and enhance current treatment of lung cancer in order to improve disease control, quality of life, symptom management, and ultimately, to make a difference in clinical outcomes and survival.
RESOURCES
Cancer and complementary health approaches (National Center for Complementary and Integrative Health)
Early detection, diagnosis, and staging
GO2 Foundation for Lung Cancer
Lung cancer (American Cancer Society)
Lung cancer (American Lung Association)
Lung cancer (National Cancer Institute)
Lung cancer information for health care providers (CDC)
Lung cancer survivors support group
Lung Helpline and Tobacco Quitline (American Lung Association)
1-800-LUNGUSA (501-1068)
Office of Cancer Complementary and Alternative Medicine (OCCAM)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
Aghdam N, McGunigal M, Wang H, Repka MC, Mete M, Fernandez S, Dash C, Al-Refaie WB, & Unger KR. (2020). Ethnicity and insurance status predict metastatic disease presentation in prostate, breast, and non-small cell lung cancer. Cancer Medicine. doi:10.1002/cam4.3109
Al-Ameri M, Sachs E, Sartipy U, & Jackson V. (2019). Uniportal versus multiportal video-assisted thoracic surgery for lung cancer. Journal of Thoracic Disease, 11(12), 5152–61. doi:10.21037/jtd.2019.12.01
American Cancer Society (ACS). (2021a). Cancer facts and figures. Retrieved from https://www.cancer.org
American Cancer Society (ACS). (2021b). Signs and symptoms of cancer. Retrieved from https://www.cancer.org
American Cancer Society (ACS). (2021c). Immunotherapy for non-small cell lung cancer. Retrieved from https://www.cancer.org
American Cancer Society (ACS). (2020a). Radiation therapy for non-small cell lung cancer. Retrieved from https://www.cancer.org
American Cancer Society (ACS). (2020b). Chemotherapy and non-small cell lung cancer. Retrieved from https://www.cancer.org
American Cancer Society (ACS). (2020c). Nausea and vomiting caused by chemotherapy. Retrieved from https://www.cancer.org
American Lung Association (ALA). (2021). Staying healthy with lung cancer. Retrieved from https://www.lung.org
American Lung Association (ALA). (2020a). What causes lung cancer? Retrieved from https://www.lung.org
American Lung Association (ALA). (2020b). Climate change and air pollution. Retrieved from https://www.lung.org
American Lung Association (ALA). (2020c). Lung cancer immunotherapy. Retrieved from https://www.lung.org
American Occupational Therapy Association (AOTA). (2021). Occupational therapy’s role in sleep. Retrieved from https://www.aota.org
American Occupational Therapy Association (AOTA). (2020). The role of occupation therapy in palliative and hospice care. Retrieved from https://www.aota.org
Bellomo C, Pixley AJ, & Johnston D. (2019). Filling an educational void: AONN+ announces cancer advocacy & patient education (CAPE) lung cancer initiative. Journal of Oncology Navigation & Survivorship, 10(12), 532–8.
Blaes AH, Adamson PC, Foxhall L, & Bhatia S. (2020). Survivorship care plans and the Commission on Cancer standards: the increasing need for better strategies to improve the outcome for survivors of cancer. JCO Oncology Practice, 16(8), 447–50.
Braveman B, Hunter EG, Nicholson J, Arbesman M, & Lieberman D. (2017). Occupational therapy interventions for adults with cancer. American Journal of Occupational Therapy, 71(5), 1–5. doi:10.5014/ajot.2017.715003
Canadian Cancer Society. (2021). Lung function tests. Retrieved from https://www.cancer.ca
Cancer Treatment Centers of America (CTCA). (2021). What is an arterial blood gas test? Retrieved from https://www.cancercenter.com
Casal RF, Walsh G, McArthur M, et al. (2018). Bronchoscopic laser interstitial thermal therapy: an experimental study in normal porcine lung parenchyma. Journal of Bronchology Interventional Pulmonology, 25(4), 322–9. doi:10.1097/LBR.0000000000000501
Centers for Disease Control and Prevention (CDC). (2021a). Secondhand smoke (SHS) facts. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021b). What are the risk factors for lung cancer? Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2021c). Lung cancer statistics. Retrieved from https://www.cdc.gov
Center for Disease Control and Prevention (CDC). (2021d). Who should be screened for lung cancer. Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2019a). What is lung cancer? Retrieved from https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2019b). CDC cancer genomic project. Retrieved from https://www.cdc.gov
Chatron E, Dégot T, Salvaterra E, Dumazet A, Porzio M, Hirschi S, Schuller A, Massard G, Renaud-Picard B, & Kessler R. (2019). Lung cancer after lung transplantation: an analysis of 25 years of experience in a single institution. Clinical Transplantation, 33(1), e13446. doi:10.1111/ctr.13446
Christensen MS, Hansen J, Ramlau-Hansen CH, Toft G, Kolstada H, & Kolstad H. (2017). Cancer incidence in workers exposed to styrene in the Danish-reinforced plastics industry, 1968–2012. Epidemiology, 28(2), 300.
Cleveland Clinic Foundation. (2020). Guided imagery. Retrieved from https://my.clevelandclinic.org
Cleveland Clinic Foundation. (2018). COPD: conserving your energy: management and treatment. Retrieved from https://my.clevelandclinic.org
Consumer Advocates for Smoke-free Alternatives Association (CASAA). (2020). A historical history for electronic cigarettes. Retrieved from http://www.casaa.org
Dartmouth-Hitchcock Norris Cotton Cancer Center. (2020). Respiration-gated radiation therapy. Retrieved from https://cancer.dartmouth.edu
Deguchi H, Tomoyasu M, Shigeeda W, Kaneko Y, Kanno H, & Saito H. (2019). Influence of prophylactic antibiotic duration on postoperative pneumonia following pulmonary lobectomy for non-small cell lung cancer. Journal of Thoracic Disease, 11(4), 1155–64. doi:10.21037/jtd.2019.04.43
Dehghani M, Keshtgar L, Javaheri MR, Derakhshan Z, Conti GO, Zucarrello P, & Ferrante M. (2017). The effects of air pollutants on the mortality rate of lung cancer and leukemia. Molecular Medicine Reports, 15(5), 3390.
Duke University. (2020). Energy conservation. Retrieved from https://sites.duke.edu
Eapen MS, McAlinden KD, Myers S, Lu W, & Sohal SS. (2019). MicroRNAs are key regulators in chronic lung disease: exploring the vital link between disease progression and lung cancer. Journal of Clinical Medicine, 8(11), 1986. doi:10.3390/jcm8111986
Eldridge L. (2020) Where does lung cancer spread? Retrieved from https://www.verywellhealth.com
Environmental Protection Agency (EPA). (2020). Drinking water requirements for states and public water systems. Retrieved from https://www.epa.gov
Gershman E, Guthrie R, Swiatek K, & Shojaee S. (2019). Management of hemoptysis in patients with lung cancer. Annals of Translational Medicine, 7(15), 358. doi:10.21037/atm.2019.04.91
GOLD. (2019). Global initiative for chronic obstructive lung disease. Retrieved from https://goldcopd.org
González J, Henschke CI, Yankelevitz DF, Seijo LM, Reeves AP, Yip R, Xie Y, et al. (2019). Emphysema phenotypes and lung cancer risk. PLoS ONE, 14(7), 1–11. doi:/10.1371/journal.pone.0219187
Green C & McGinley PC. (2019). Yielding to the necessities of a great public industry: denial and concealment of the harmful health effects of coal mining. William & Mary Environmental Law & Policy Review, 43(3), 689–757.
Haider T, Arshad AR, Arshad A, & Tufail M. (2021). Lung cancer services and the COVID-19 pandemic. Annals of King Edward Medical University, 27(1), 146–9.
Hakoda H, Sekine Y, Ichimura H, Ueda K, Aoki S, & Sato Y. (2019). Hepatectomy for rapidly growing solitary liver metastasis from non-small cell lung cancer: a case report. Surgical Case Reports, 5(1), 1–5. doi:10.1186/s40792-019-0633-6
Han Y, Zhang Y, Li C, Yang S, & Li H. (2020). Robotic lung cancer surgery: from simple to complex, from surgery to clinical study. Journal of Thoracic Disease, 12(2), 51–3. doi:10.21037/jtd.2019.09.79
Harding MM, Kwong J, Roberts D, Hagler D, & Reinisch C. (2020). Lewis’s medical-surgical nursing: assessment and management of clinal problems (11th ed.). Elsevier.
Hayes JT. (2017). What is third-hand smoke and why is it a concern? Retrieved from https://www.mayoclinic.org
Healthline. (2021a). Early signs and symptoms of lung cancer. Retrieved from https://www.healthline.com
Healthline. (2021b). Your FAQs answered: lung cancer and white blood cell count. Retrieved from https://www.healthline.com
Heo JW, Yeo CD, Park CK, Kim SK, Kim JS, Kim JW, Kim SJ, Lee SH, & Kang HS. (2020). Smoking is associated with pneumonia development in lung cancer patients. BMC Pulmonary Medicine, 20(1), 117. doi:10.1186/s12890-020-1160-8
Hilzenrat RA, Deen SA, Yee J, Grant KA, Ashrafi AS, Coughlin S, & McGuire AL. (2021). Thoracic surgeon impressions of the impact of the COVID-19 pandemic on lung cancer care-lessons from the first wave in Canada. Current Oncology, 28(1), 940–9. doi:10.3390/curroncol28010092
Hospice Alliance. (2020). Hospice vs. palliative care. Retrieved from https://www.hospicealliance.org
Howley EK. (2019). A patient’s guide to lung cancer. Retrieved from https://health.usnews.com
Hunter EG, Gibson RW, Arbesman M, & D’Amico M. (2017a). Centennial topics—systematic review of occupational therapy and adult cancer rehabilitation: part 1. Impact of physical activity and symptom management interventions. American Journal of Occupational Therapy, 71, 7102100030. doi:10.5014/ajot.2017.023564
Hunter EG, Gibson RW, Arbesman M, & D’Amico M. (2017b). Centennial topics—systematic review of occupational therapy and adult cancer rehabilitation: part 2. Impact of multidisciplinary rehabilitation and psychosocial, sexuality, and return-to-work interventions. American Journal of Occupational Therapy, 71, 7102100040. doi:10.5014/ajot.2017.023572
Ironwood Cancer & Research Center. (2020). What is integrative oncology? Retrieved from https://www.ironwoodcrc.com
Jin C & Lang B. (2019). Hormone replacement therapy and lung cancer risk in women: a meta-analysis of cohort studies. Medicine, 98(51), e17532. doi:10.1097/MD.0000000000017532
Johns Hopkins Medicine (JHM). (2020a). What are pulmonary function tests? Retrieved from https://www.hopkinsmedicine.org
Johns Hopkins Medicine (JHM). (2020b). Acupuncture. Retrieved from https://www.hopkinsmedicine.org
Journy N, Mansouri I, Allodji RS, Demoor-Goldschmidt C, Ghazi D, Haddy N, Rubino C, Veres C, Zrafi WS, Rivera S, Diallo I & De Vathaire F. (2019). Volume effects of radiotherapy on the risk of second primary cancers: A systematic review of clinical and epidemiological studies. Radiotherapy & Oncology, 131, 150–9. Retrieved from https://doi.org/10.1016/j.radonc.2018.09.017
Juckett LA & Robinson ML. (2019). Occupational therapy approach to addressing food insecurity among older adults with chronic disease. Geriatrics (Basel, Switzerland), 4(1), 22. doi:10.3390/geriatrics4010022
Kawaguchi T, Sawabata N, Miura S, Kawai N, Yasukawa M, Tojo T, & Taniguchi S. (2019). Prognostic impact of underlying lung disease in pulmonary wedge resection for lung cancer. International Journal of Clinical Oncology, 24(4), 366–74. doi:10.1007/s10147-018-1367-3
Kendall F, Abreu P, Pinho P, Oliveira J, & Bastos P. (2017). The role of physiotherapy in patients undergoing pulmonary surgery for lung cancer: a literature review. Portuguese Journal of Pulmonology, 23(6), 343–51. doi:10.1016/j.rppnen.2017.05.003
Lai Y, Huang J, Yang M, Su J, Liu J, & Che G. (2017). Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. Journal of Surgical Research, 209, 30–6. doi:10.1016/j.jss.2016.09.03
LungCancer.org. (2020). Managing cancer pain. Retrieved from https://www.lungcancer.org
Mayo Clinic (2022). Stereotactic body radiotherapy. Retrieved from https://www.mayoclinic.org
Mayo Clinic. (2020a). Emphysema. Retrieved from https://www.mayoclinic.orgsyc-20355555
Mayo Clinic. (2020b). Video-assisted thoracoscopic surgery (VATS). Retrieved from https://www.mayoclinic.org
Mayo Clinic. (2020c). Massage: get in touch with its many benefits. Retrieved from https://www.mayoclinic.org
Mazzei M & Abbas AE. (2020). Why comprehensive adoption of robotic assisted thoracic surgery is ideal for both simple and complex lung resections. Journal of Thoracic Disease, 12(2), 70–81. https://doi.org/10.21037/jtd.2020.01.22
MedlinePlus. (2020a). Lung cancer. Retrieved from https://medlineplus.gov
MedlinePlus. (2020b). Mediastinoscopy with biopsy. Retrieved from https://medlineplus.gov
Molassiotis A, Smith JA, Mazzone P, Blackhall F, & Irwin RS. (2017). Symptomatic treatment of cough among adult patients with lung cancer: CHEST guideline and expert panel report. Chest, 151(4), 861–74. doi:10.1016/j.chest.2016.12.028
Molina-Romero C, Arrieta O, & Hernández-Pando R. (2019). Tuberculosis and lung cancer. Salud Pública de México, 61(3), 286–91. doi:10.21149/10090
Nakamura A, Kondo N, Nakamichi T, Hashimoto M, Takuwa T, Matsumoto S, Kuribayashi K, Kijima T & Hasegawa S. (2021). Complications and predictive factors for air leak >10 days with neoadjuvant chemotherapy followed by pleurectomy/decortication for malignant pleural mesothelioma. Annals of Surgical Oncology, 28(6), 3057–65. Retrieved from https://doi.org/10.1245/s10434-020-09275-y
Natera. (2021). Signatera. Retrieved from https://www.natera.com
National Cancer Institute (NCI). (2022). Crizotinib in treating patients with stage IB-IIIA non-small cell lung cancer that has been removed by surgery and ALK fusion mutations (an ALCHEMIST treatment trial). Retrieved from https://www.cancer.gov
National Cancer Institute (NCI). (2021). Targeted cancer therapies. Retrieved from https://www.cancer.gov
National Cancer Institute (NCI). (2020a). NCI dictionary of cancer terms. Retrieved from https://www.cancer.gov
National Cancer Institute (NCI). (2020b). Brachytherapy to treat cancer. Retrieved from https://www.cancer.gov
National Cancer Institute (NCI). (2020c). External beam radiation therapy for cancer. Retrieved from https://www.cancer.gov
National Cancer Institute (NCI). (2019). Metastatic cancer. Retrieved from https://www.cancer.gov
National Comprehensive Cancer Network (NCCN). (2021). Small cell lung cancer. Retrieved from https://www.nccn.org
National Comprehensive Cancer Network (NCCN). (2019). Non-small cell lung cancer early and locally advanced. Retrieved from https://www.nccn.org
National Health Service (NHS). (2019). Lambert-Eaton myasthenic syndrome. Retrieved from https://www.nhs.uk
National Institutes of Health (NIH). (2020a). Yoga: what you need to know. Retrieved from https://www.nccih.nih.gov
National Institutes of Health (NIH). (2020b). Lung cancer. Retrieved from https://ghr.nlm.nih.gov
Niu M, Yi M, Li N, Luo S, & Wu K. (2021). Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Experimental Hematology & Oncology, 10(18). Retrieved from http://doi.org/10.1186/s40164-021-00211-8
Nurse.org. (2020). Know your ABGs: arterial blood gases explained. Retrieved from https://nurse.org
Passaro A, Bestvina C, Velez Velez M, et al. (2021). Severity of COVID-19 in patients with lung cancer: evidence and challenges. Journal for ImmunoTherapy of Cancer. Retrieved from https://jitc.bmj.com
Peng Y, Liu Q, Wang Y, Song A, Duan H, Qiu Y, Li Q, & Cui H-J. (2019). Pathological diagnosis and treatment outcome of gastric metastases from small cell lung cancer: a case report. Oncology Letters, 18(2), 1999–2006. doi:10.3892/ol.2019.10484
Pesch B, Kendzia B, Pohlabeln H, Ahrens W, Wichmann H-E, Siemiatycki J, Taeger D, et al. (2019). Exposure to welding fumes, hexavalent chromium, or nickel and risk of lung cancer. American Journal of Epidemiology, 188(11), 1984–93. doi:10.1093/aje/kwz187
Petit P, Maître A, Persoons R, & Bicout DJ. (2019). Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environment International, 124, 109–20. doi:10.1016/j.envint.2018.12.058
Physiopedia. (2022). Physiotherapy in palliative care. Retrieved from https://www.physio-pedia.com
Physiopedia. (2020). Lung anatomy. Retrieved from https://www.physio-pedia.com
Poole P, Sathananthan K, & Fortescue R. (2019). Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews, 5, CD001287. doi:10.1002/14651858.CD001287.pub6
Potter PA, Perry AG, Stockert PA, & Hall AM. (2019). Fundamentals of nursing (10th ed.). Elsevier.
Rha SY, Park JS, Choi YY, Hong B, & Lee J. (2022). Fear of cancer recurrence and its predictors and outcomes among cancer survivors: a descriptive correlational study. European Journal of Oncology Nursing, 58. Retrieved from https://doi.org/10.1016/j.ejon.2022.102138
Sanchez-Carpintero Abad M, Sanchez-Salcedo P, de-Torres JP, Alcaide AB, Seijo LM, Pueyo J, Bastarrika G, Zulueta JJ, & Campo A. (2020). Prevalence and burden of bronchiectasis in a lung cancer screening program. PLoS ONE, 15(4), 1–11. doi:10.1371/journal.pone.0231204
Schneider BJ, Ismaila N, Aerts J, Chiles C, Daly ME, et al. (2019). Lung cancer surveillance after definitive curative-intent therapy. American Society of Clinical Oncology. Retrieved from https://www.asco.org
Shtraichman O & Ahya VN. (2020). Malignancy after lung transplantation. Annals of Translational Medicine, 8(6), 416. Retrieved from https://doi.org/10.21037/atm.2020.02.126
Siddiqui F, Vaqar S, & Siddiqui A. (2021). Lung cancer. Retrieved from https://www.ncbi.nlm.nih.gov
Song P, Zhang X, Yang D, Wang H, Si X, & Zhang L. (2020). Single—center study to determine the safety and efficacy of CT—707 in Chinese patients with advanced anaplastic lymphoma kinase—rearranged non—small—cell lung cancer. Thoracic Cancer, 11(5), 1216–23. doi:10.1111/1759-7714.13376
Tan W. (2019). Which paraneoplastic syndromes are associated with small cell lung cancer (SCLC)? Medscape. Retrieved from https://www.medscape.com
Taylor SF. (2018). Occupational therapy and the cancer care: adjusting treatment focuses. Retrieved from https://www.aota.org
Teng X, Wei L, Han L, Min D, & Du Y. (2020). Establishment of a serological molecular model for the early diagnosis and progression monitoring of bone metastasis in lung cancer. BMC Cancer, 20(1), 1–11. doi:10.1186/s12885-020-07046-2
Thronicke A, von Trott P, Kröz M, Grah C, Matthes B, & Schad F. (2020). Health-related quality of life in patients with lung cancer: applying integrative oncology concepts in a certified cancer centre. Evidence-Based Complementary & Alternative Medicine (ECAM), 1–9. doi:/10.1155/2020/3543568
Tolwin Y, Gillis R, & Peled N. (2020). Gender and lung cancer—SEER-based analysis. Annals of Epidemiology, 46, 14–9. doi:10.1016/j.annepidem.2020.04.003
Ueda K, Murakami J, Tanaka T, Yoshida K, Kobayashi T, & Hamano K. (2018). Predicting the response to a bronchodilator in patients with airflow obstruction and lung cancer. Journal of Surgical Research, 228, 20–6. doi:10.1016/j.jss.2018.02.016
University of Rochester Medical Center (URMC). (2020). Pulmonary function tests: what are pulmonary function tests? Retrieved from https://www.urmc.rochester.edu
University of San Diego Health (USDH). (2021). Endobronchial ultrasound bronchoscopy (EBUS). Retrieved from https://health.ucsd.edu
University of San Diego Health (USDH). (2020). Tracheobronchial airway stent placement. Retrieved from health.ucsd.edu/specialties/pulmonary/procedures/Pages/airway-stent.aspx
Verywell Health. (2021). What is lung cancer recurrence. Retrieved from https://www.verywellhealth.com
Vuong A. (2020). Eradicating harmful particles in the workplace. Professional Safety, 65(5), 44–5.
White KM. (2016). The role of the occupational therapist in the care of people living with lung cancer. Transl Lung Cancer Res, 5(3), 244–6. doi:10.21037/tlcr.2016.0
World Health Organization (WHO). (2018). Ambient (outdoor) air pollution. Retrieved from https://www.who.int
Wu S-Y, Xing F, Sharma S, Wu K, Tyagi A, Liu Y, et al. (2020). Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med, 217(8), e20191131. doi:10.1084/jem.20191131
Zaatar M, Stork T, Valdivia D, Mardanzai K, Stefani D, Collaud S, Poellen P, Hegedus B, Ploenes T, & Aigner C. (2020). Minimal invasive approach reduces cardiopulmonary complications in elderly after lung cancer surgery. Journal of Thoracic Disease, 12(5), 2372–9. doi:10.21037/jtd.2020.03.73
Zablotska LB, Fenske N, Schnelzer M, Zhivin S, Laurier D, & Kreuzer M. (2018). Analysis of mortality in a pooled cohort of Canadian and German uranium processing workers with no mining experience. International Archives of Occupational and Environmental Health, 91(1), 91–103. doi:10.1007/s00420-017-1260-9
Zaborowska-Szmit M, Krzakowski M, Kowalski DM, & Szmit S. (2020). Cardiovascular complications of systemic therapy in non-small cell lung cancer. Journal of Clinical Medicine, 9(5), 1268. doi:10.3390/jcm9051268
Zeng Y, Jiang F, Chen Y, Chen P, & Cai. (2018). Exercise assessments and trainings of pulmonary rehabilitation in COPD: a literature review. International Journal of Chronic Obstructive Pulmonary Disease, 13, 2013–23. Retrieved from https://doi.org/10.2147/COPD.S167098





Customer Rating
5.0 / 884 ratings