Diabetes Type 2
Prevention, Symptoms, and Treatment
Online Continuing Education Course

Course Description
Type 2 diabetes continuing education. 7-contact-hour CEU course offering a comprehensive overview of DMT2, including diabetes prevention, causes, symptoms, risk factors, and treatment.
Note: This course is also available as part of a package. See Nursing CEU Bundle - 30 ANCC Hours
Course Price: $42.00
Contact Hours: 7
Pharmacotherapeutic Hours: 0.5
Course updated on
December 5, 2023
"This information was very well organized and helpful. I will take so much of it and put it into my nursing practice. Thank you for this resource!" - Erica, RN in Illiniois
"A great course. My husband has type 2 diabetes, and I learned a lot." - Christie, RN in Minnesota
"The patient care plan data was complete and is an excellent guide to follow." - Wendy, RN in Georgia
"Thank you for the ability to print out the course so I can take notes and highlight the material." - Maribeth, case manager in New Mexico
Diabetes Type 2
Prevention, Symptoms, and Treatment
Copyright © 2023 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will demonstrate increased knowledge of evidence-based guidelines for caring for persons with type 2 diabetes. Specific learning objectives to address potential knowledge gaps include:
- Describe the incidence, prevalence, costs, and groups at risk of developing type 2 diabetes.
- Review the underlying causes of diabetes.
- Discuss prevention strategies for persons at risk of developing type 2 diabetes.
- Describe the assessment and screening criteria used to diagnose patients.
- Review current goals of treatment for persons with type 2 diabetes.
- Describe the components of a diabetes self-management education plan, including lifestyle interventions and nutrition management.
- Review medications and metabolic surgery options.
- Discuss the most common and serious complications associated with type 2 diabetes and their effective treatment interventions.
TABLE OF CONTENTS
- What Is Diabetes?
- Causes of Type 2 Diabetes
- Screening and Prevention of Type 2 Diabetes
- Assessment and Diagnosis of Type 2 Diabetes
- Developing a Type 2 Diabetes Treatment Plan
- Lifestyle Changes and Self Manangement
- Medical Treatment for Type 2 Diabetes
- Hyperglycemia-Related Illnesses and Complications
- Conclusion
- Resources
- References
WHAT IS DIABETES?
Diabetes mellitus—or, simply, diabetes—is a chronic illness in which the body is exposed to continual high levels of blood glucose, a condition known as hyperglycemia. In the short term, extreme hyperglycemia can lead to life-threatening dehydration and coma. Over the long term, hyperglycemia damages capillaries and larger blood vessels by thickening their walls and narrowing their inner diameters. This reduces the blood flow to many areas of the body and causes permanent tissue damage, notably to the retinas and the kidneys. Long-term high blood glucose levels also damage nerve endings.
- An estimated 37.3 million people, or 11.3% of the U.S. population, have diabetes.
- Around 8.5 million people in the United States have undiagnosed diabetes.
- Approximately half of U.S. adults have diabetes or prediabetes.
- Each year, more Americans die from diabetes than AIDS and breast cancer combined. Diabetes is the eighth leading cause of death in the United States.
- People with diabetes have higher rates of death due to cardiovascular disease and higher rates of hospitalization for heart attacks and stroke.
- Diabetes is the number one cause of kidney failure, adult blindness, and nontraumatic lower extremity amputations.
- The vast majority of adults with diagnosed diabetes (89.8%) are overweight.
(CDC, 2022a, 2023; ADA, 2022a)
Almost all forms of diabetes stem from problems in the body’s production and use of insulin, the hormone that is responsible for keeping blood glucose levels in check. One cause of diabetes is the inability to produce enough insulin; for this problem, treatments include oral medications, noninsulin injectable medications, and insulin injections.
Another cause of diabetes is insulin resistance, which is the inability of body tissues to respond sufficiently to normal amounts of insulin; here, the treatments include exercise, weight loss, and, when needed, oral medications that increase tissue responsiveness to insulin (ElSayed et al., 2023a).
The three main types of diabetes are:
- Type 1 diabetes, which is characterized by destruction of the insulin-secreting cells (beta cells) of the pancreas
- Type 2 diabetes, which is characterized by insulin resistance and progressively reduced secretion of insulin by beta cells
- Gestational diabetes, which develops in women who are pregnant
(ADA, 2022a; CDC, 2023)
About 90% to 95% of people with diabetes have type 2 diabetes, and about 5% to 10% have type 1 diabetes. The typical patient with type 2 diabetes is an adult who has had the disease for many years before it worsens sufficiently to cause symptoms that prompt healthcare intervention (CDC, 2022a, 2023; ElSayed et al., 2023a).
People who do not have especially high levels of blood glucose but who do have inefficient (“impaired”) mechanisms for handling blood glucose have a condition called prediabetes, which is diagnosed when fasting glucose levels are elevated in the range of 101–125 mg/dL on more than one occasion (ElSayed et al., 2023a).
Currently, diabetes is incurable, and it takes daily management to prevent or delay further damage to the body. Consuming a healthy diet, participating in regular physical activity, maintaining a normal body weight, and avoiding tobacco use are ways to prevent or delay the onset of type 2 diabetes (CDC, 2023).
Complications can be prevented or hindered through consistent screenings and appropriate treatment (WHO, 2023). The most successful model for managing diabetes is a team approach. In this approach, the patient is considered to be the daily healthcare manager, and a group of professionals—including physicians, nutritionists, nurses, and other allied health professionals—act as guides, advisors, monitors, and counselors.
History of Diabetes
Type 2 diabetes is one of the two main forms of diabetes mellitus, a disease that has been a problem during all of recorded human history. Diabetes is a Greek word that means “to pass through.” Diabetes was the name given to diseases in which a person continually drinks great quantities of fluid, which then pass through the body and are excreted as great quantities of urine. Diabetes is thus characterized by polydipsia (prodigious drinking) and polyuria (prodigious urinating).
Even in early times, two different diabetes diseases were distinguished: diabetes insipidus and diabetes mellitus. People with diabetes insipidus have symptoms of dilute, watery urine. This disease is now known to be caused most often by the insufficient secretion of ADH (antidiuretic hormone) by the pituitary gland. In contrast, people with diabetes mellitus produce urine that is denser than normal and that leaves crystals of sugar when the water in the urine is evaporated. Diabetes insipidus is rare, and even before the physiologic bases of the diseases were understood, when someone spoke simply of “diabetes,” they were usually referring to diabetes mellitus.
DIABETES IN THE PAST
Before the 20th century, diabetes mellitus was usually fatal. Most often, diabetes occurred in people who were older than 50 years of age and obese. The disease came on gradually, with increasing thirst and correspondingly voluminous urination. The individual’s mouth and skin were always dry, and the breath often had a sweetish odor.
The disease progressed inexorably, bringing with it a host of problems. Eyesight failed from cataracts and nerve problems. Muscles weakened, skin infections and pneumonias were common, and people developed gangrene of the lower limbs. Diabetes led to digestive troubles, kidney disease, and heart failure. Death was usually from what was then called diabetic coma (now called diabetic ketoacidosis), which came on suddenly and was always fatal within a few days.
In the less-common cases in which children, teenagers, or young adults developed diabetes, the disease worsened much more rapidly. There were no good treatments for diabetes, although a low-carbohydrate diet slowed the progression of the disease in some individuals with obesity who developed the disease later in life.
THE DISCOVERY OF INSULIN
By the early 1800s, pancreatic damage was recognized in autopsies of people who died of diabetes, and late in that century German scientists showed that removing the pancreas from a dog would cause diabetes in the animal. However, diabetes could be prevented in these dogs if a piece of pancreas was sewn under the dog’s skin, and this suggested that the pancreas made a substance that prevented diabetes.
Attempts to extract this substance failed because the pancreas also makes a number of destructive enzymes, whose presence in the extracts would destroy the key antidiabetes substance. In the early 1920s, the Canadian surgeon Frederick Banting and his assistant Charles Best, a medical student, devised a way to rid the pancreas of most of its destructive enzymes. They extracted a hormone from the pancreatic tissue that would decrease the amount of sugar in the bloodstream and in the urine of diabetic dogs. They named this antidiabetes hormone insulin. Before the discovery and purification of insulin, diabetes was a fatal disease; after Banting and Best’s work, diabetes became a chronic illness (Ghosh et al., 2022).
Identifying the Two Types of Diabetes
At the beginning of the 20th century, diabetes mellitus was considered one disease, although young people who developed the disease died much more quickly than people who first became ill in middle or old age. The new treatment with insulin, however, began to highlight a number of other differences. As early as the 1930s, clinicians found that people with diabetes could be divided into two classes according to the way they reacted to an injection of insulin.
People with insulin-sensitive diabetes (who tended to be young and prone to developing ketosis, a buildup of ketone bodies in body tissues and fluids, leading to nausea, vomiting, and stomach pain) easily disposed of an oral dose of glucose after receiving an injection of insulin. In contrast, people with insulin-insensitive diabetes (who were usually middle-aged and did not have ketotic episodes) did not significantly reduce their blood glucose levels after receiving the same amount of insulin.
TYPE 1 DIABETES
Type 1 diabetes was previously called juvenile diabetes or insulin-dependent diabetes. In type 1 diabetes, the pancreas produces little or no insulin because the beta cells (the insulin-making endocrine cells in the islets of Langerhans of the pancreas) are not functioning. This is thought to be due to an autoimmune process that occurs in the beta cells. Type 1 diabetes occurs most commonly in young people, although it can occur in any age group (ElSayed et al., 2023a).
TYPE 2 DIABETES
Type 2 diabetes occurs most often in older adults, although it can occur at any age. Type 2 diabetes develops when the tissues in the body become resistant to insulin that is made by the pancreas or when the pancreas is unable to produce enough insulin. This is called insulin resistance (ElSayed et al., 2023a).
The majority of people with diabetes have the type 2 form, previously called insulin-insensitive diabetes, non-insulin-dependent diabetes, or adult-onset diabetes. In type 2 diabetes, the pancreas often produces enough insulin to prevent ketone (a chemical produced in the liver when fat is used for energy) formation but, because of insulin resistance, not enough to prevent hyperglycemia.
Although there is a hereditary (i.e., genetic) predisposition for the disease, type 2 diabetes does not appear to have a single cause. Aging, a sedentary lifestyle, and obesity can activate or enhance a person’s predisposition to develop type 2 diabetes (ElSayed et al., 2023a).
Type 2 diabetes worsens quickly if it is not treated. Both hyperglycemia and higher-than-normal circulating insulin levels (hyperinsulinemia) increase the existing insulin resistance. Hyperglycemia also injures the beta cells (the insulin-manufacturing cells) in the pancreas, and this makes it increasingly difficult for the pancreas to lower high levels of blood glucose. As these processes continue and interact with each other, the person with diabetes has more frequent and higher episodes of hyperglycemia, which over time damage the eyes, kidneys, nerves, and blood vessels (ElSayed et al., 2023a).
Incidence and Prevalence of Type 2 Diabetes
Type 2 diabetes is now considered a worldwide epidemic. The U.S. Centers for Disease Control and Prevention estimates that over 37 million Americans have diabetes. The disease affects 11.3% of all Americans. Among people ages 18 years or older, 96 million (over one third of the U.S. adult population) also have prediabetes. The National Diabetes Statistics Report for 2022 reveals higher rates of diabetes among the non-Hispanic Black U.S. population compared to the general population (CDC, 2022a).
Worldwide, the number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014. Additional statistics published by the WHO offer an alarming picture of diabetes throughout the world. These data include:
- From 2000 to 2019, mortality rates from diabetes increased by 3%.
- In 2019, an estimated 1.5 million deaths were directly caused by diabetes.
- Diabetes prevalence has been rising more rapidly in middle- and low-income countries.
- Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke, and lower limb amputation.
- Almost half of all deaths attributable to high blood glucose occur before the age of 70 years.
(WHO, 2023)
DIABETES BY AGE AND RACE
Diabetes is more common in older people. According to the CDC, 29.2% of people aged 65 years or older have diabetes. The rate of diabetes also varies by race. In the United States, diabetes is more common among non-Whites than Whites. After adjusting for population age differences, 2017–2020 national survey data for people ages 18 years or older reveal the following prevalence rates for diagnosed diabetes:
- 12.7% of non-Hispanic Blacks
- 11.1% of Hispanics
- 11.3% of Asian Americans
- 11.0% of non-Hispanic Whites
(CDC, 2022a)
The CDC continues to gather data and update information regarding the incidence and prevalence of diabetes and prediabetes in the United States. Additional data from 2019 include the following:
- 1.4 million new cases of diabetes were diagnosed.
- Compared to adults age 18 to 44 years, incidence rates of diagnosed diabetes were higher among adults ages 45 to 64 years and in adults ages 65 years and older.
- Non-Hispanic Blacks (6.5 per 1,000 persons) and people of Hispanic origin (7.0 per 1,000 persons) had a higher incidence compared to non-Hispanic Whites (6.0 per 1,000 persons).
(CDC, 2022a)
CHILDREN AND ADOLESCENTS
In the past two decades, type 2 diabetes has been reported among children and adolescents in the United States with an increasing frequency. The epidemic of obesity, the low level of physical activity among young people, and exposure to diabetes in utero may be contributing factors.
Children diagnosed with type 2 diabetes are usually between 10 and 19 years old, obese, and have a strong family history for type 2 diabetes. While the prevalence of type 2 diabetes is increasing in children of all ethnic groups, it has increased more significantly in non-Hispanic Black children.
Obesity and sedentary lifestyle are key factors driving the dramatic increase of type 2 diabetes in our society. Type 2 diabetes is associated with increased associated comorbid conditions and early mortality (CDC, 2022a; ElSayed et al., 2023a).
The increase in prevalence of type 2 diabetes among children and adolescents is a new challenge for healthcare providers and the health system to monitor and manage. New strategies for prevention, early detection, and treatment must be developed and implemented as this new generation of people with type 2 diabetes matures. As these individuals enter their adult years, they may have unique health challenges and may be at risk for developing early complications because of the early onset of the disease. This group may also have an increase in the frequency of diabetes during the reproductive years, which may further increase diabetes incidence in the next generation (ElSayed et al., 2023a).
Costs of Diabetes
The impact of diabetes is significant in monetary terms. Costs related to care and complications of the disease are dramatic and include the following factors:
- In 2017 the total economic burden in the United States of diagnosed diabetes was $327 billion, including $237 billion in direct costs and $90 billion in indirect costs (e.g., disability, early death, lost work time).
- Approximately 25% of all healthcare dollars are spent taking care of people with diabetes.
- People with diagnosed diabetes have healthcare costs over 2.3 times higher than what expenditures would be in the absence of diabetes.
- The cost of diabetes medications (notably insulin) is an ongoing barrier to treatment. The price of insulin almost tripled from 2002 to 2013. Up to 25% of patients who are prescribed insulin report not using it because of cost barriers.
- More than 60% of nontraumatic lower limb amputations occur in people with diabetes.
(ADA, 2022a; ElSayed et al., 2023b)
CAUSES OF TYPE 2 DIABETES
The etiology of type 2 diabetes is believed to be the result of complex interactions between environmental and genetic factors. The disease develops in response to a diabetes-prone lifestyle (i.e., excessive caloric intake, obesity, lack of exercise) in conjunction with a susceptible genotype.
Normal Glucose Metabolism
Since diabetes is associated with abnormal levels of blood glucose, it is useful to understand how the body metabolizes glucose.
WHAT IS GLUCOSE?
Carbohydrates come in all sizes. Large carbohydrates, such as polysaccharides (e.g., starch), are chains of individual sugar molecules. The smallest carbohydrates are monosaccharides, or individual sugar molecules. Glucose, which is a small, water-soluble molecule, is a monosaccharide.
Glucose is an essential molecule, but most tissues of the body can survive when there are low levels of blood glucose. The brain, however, is quite sensitive to low blood glucose, and it suffers irreversible damage if hypoglycemia lasts more than about half an hour. The dependence of the brain on continuous supplies of glucose makes it crucial that the body maintain sufficient blood glucose levels at all times.
WHAT IS GLYCOGEN?
Excess blood glucose is stored in the liver and muscles as long chains (polysaccharides) called glycogens. After a meal, insulin in the bloodstream lowers the amount of circulating glucose by encouraging its storage in the form of glycogen molecules. Between meals, liver glycogen is broken down to maintain sufficient glucose in the bloodstream, and the production of glucose from glycogen is encouraged by another pancreatic enzyme, glucagon.
In this way, two pancreatic hormones—insulin and glucagon—balance the amount of glucose in the bloodstream: insulin lowers the level of plasma glucose by encouraging liver cells to take up glucose and store it in the form of glycogen, while glucagon raises the level of plasma glucose by encouraging the liver to break down stored glycogen and release the resulting glucose molecules (Nakrani et al., 2023).
THE ROLE OF INSULIN
Glucose is the primary stimulus for insulin secretion. The pancreas releases insulin in response to the blood levels of amino acids or when signaled by the parasympathetic (vagal activity) nervous system. Insulin is continuously released from the pancreas into the bloodstream. Even though insulin is rapidly destroyed within five to six minutes, the impact on body cells may last as long as one to one and a half hours (Diabetes Education Online, 2023).
Insulin is the only hormone that works to significantly reduce the blood glucose level. Insulin accomplishes this by facilitating the entry of glucose from the bloodstream into the cells, which promotes glycogenesis and stimulates glucose catabolism.
It is important to note that a number of factors can affect fasting blood glucose levels. These include, but are not limited to:
- Low potassium levels, since potassium is used in beta cell depolarization
- Antipsychotic drugs, corticosteroids, statins, and thiazide diuretics, which may cause increased levels
- Alcohol, antibiotics such as levofloxacin and trimethoprim-sulfamethoxazole, indomethacin, and propranolol overdose, which may cause decreased levels
(Ridwanto et al., 2020; Diabetes.co.uk., 2023a; MedlinePlus, 2023)
There are many types of medications that can affect the results of glucose testing, and the preceding list is not all-inclusive. Therefore, it is important to know which medications a patient is taking and to check if such medications interfere with blood glucose test results.
NORMAL INSULIN SECRETION
Under normal conditions, insulin molecules circulating in the bloodstream bind to receptors located on the cells of the body. When activated by insulin, portals open to allow glucose to enter the cells, where it is converted to energy.
In the pancreas, nests of cells referred to as islets of Langerhans contain both beta and alpha cells. The majority of the cells are beta cells, which produce and store insulin until needed. The remaining cells, alpha cells, make and store glucagon, the hormone that counteracts the effects of insulin.
Normally, pancreatic hormonal secretion is perfectly balanced. Beta and alpha cells monitor blood glucose levels on an ongoing basis and release insulin or glucagon as appropriate. In diabetes, however, beta cells secrete inadequate amounts of insulin (or sometimes no insulin at all). Thus, glucose is unable to enter the cells of the body, and the necessary fuel for energy production remains ineffectively in the bloodstream.
Genetic Causes
Some aspects of all these predisposing problems are inherited, and in this way, the propensity for developing type 2 diabetes is inherited. The specific genetic causes are not known in detail for most variants of type 2 diabetes, but most cases appear to be polygenic (involving more than one inherited problem) (ElSayed et al., 2023a).
The genetics of type 2 diabetes are not completely known. They are complex, and current evidence suggests that multiple genes in pancreatic beta cell failure and insulin resistance are involved. Specifically identified genetic variants account for about 10% of the heritable component of most cases of type 2 diabetes.
Some forms of diabetes have an evident link to genetic abnormalities. The syndrome historically known as maturity onset diabetes of the young (MODY) is now known to be caused by a variety of defects in beta cell function. This accounts for less than 5% of persons with type 2 diabetes who present at a young age and have only mild disease (ElSayed et al., 2023a).
Diabetes Secondary to Other Conditions
Some health conditions can lead to the development of secondary diabetes. Diabetes is a common comorbidity with cystic fibrosis. Approximately 40% to 50% of adults with cystic fibrosis also have cystic fibrosis–related diabetes.
Diabetes can also develop after organ transplantation. This is primarily due to hyperglycemia caused by stress, steroids, and immunosuppressive regimens necessary to prevent organ rejection (ElSayed et al., 2023a).
Other health conditions that can result in secondary diabetes include:
- Hemochromatosis
- Chronic pancreatitis
- Polycystic ovary syndrome (PCOS)
- Cushing’s syndrome
- Pancreatic cancer
- Glucagonoma
- Pancreatectomy
(Diabetes.co.uk., 2023b)
Insulin Resistance
Insulin resistance is a molecular problem in which most tissues do not respond normally to insulin in the bloodstream, whether the insulin has been secreted by the pancreas or has been administered therapeutically.
Insulin resistance is the predominant factor that leads to type 2 diabetes, gestational diabetes, and prediabetes. When the body becomes resistant to insulin, it attempts to compensate by producing more insulin. Thus, individuals with insulin resistance are frequently producing more insulin than those who are healthy. Producing too much insulin is referred to as hyperinsulinemia.
Some research shows that insulin resistance can be reduced by:
- Following low-carbohydrate and ketogenic diets
- Following low-calorie diets
- Surgery for weight loss
- Exercise combined with a healthy diet
(Diabetes.co.uk, 2022)
A ketogenic diet is a low-carbohydrate diet in which most of the calories come from fat and protein sources. This type of diet alters the way energy is used in the body. Fat is converted into fatty acids and ketone bodies. This helps to improve glycemic control and reduce the fasting blood glucose level. Results from a meta-analysis concluded that a ketogenic diet might improve insulin resistance through weight reduction (Yuan et al., 2020).
EFFECTS OF INSULIN RESISTANCE
In a person with insulin resistance, a normal amount of circulating insulin produces:
- Less than the normal amount of glucose transport into cells
- Less than the normal use of intracellular glucose
- Less than the normal storage of glucose in the form of glycogen
- More than the normal release of glucose into the circulation by the liver
Most people with type 2 diabetes have insulin resistance. Insulin resistance can exist in a person years before the diabetes is diagnosed, and the presence of insulin resistance in an asymptomatic person predicts the high probability of developing type 2 diabetes. Although diabetes is often thought of as a disease of the pancreas, insulin resistance is a problem in the cells throughout the body that respond to insulin. Usually, it is a problem in the molecular mechanisms by which cells recognize the insulin molecule and then produce the intracellular effects of this recognition.
While the exact causes of insulin resistance are unknown, there are many separate molecular sites that could be the source of insulin resistance. Insulin receptors (which are in the membranes of responding cells) are complex structures made of a number of separate subunits. The malfunctioning or mutation of any of these subunits can make them work inefficiently or make them insensitive to insulin, leading to insulin resistance. Insulin resistance can also be caused by the malfunctioning of any of the components of the intracellular cascade that connects the insulin receptors in the cell membrane to the glucose-processing machinery inside the cell. In addition to having hyperinsulinemia, people with insulin resistance have been found to have high inflammation levels and a surplus of fat stored in the liver and pancreas (Diabetes.co.uk, 2022).
EXCESS VISCERAL FAT
Intra-abdominal fat is strongly associated with insulin resistance—more so than is extra-abdominal (subcutaneous) fat. Intra-abdominal fat is visceral fat, and an overabundance of visceral fat cells both triggers and worsens insulin resistance (Wondmkun, 2020).
About 90% of body fat is subcutaneous fat, which is the kind of fat felt when the skin is pinched. The remaining 10% is intra-abdominal fat, which is located beneath the abdominal muscles and can only be detected by MRI (Cleveland Clinic, 2022).
Signals within the sympathetic nervous system cause fat cells to break down and release their stored fat. Insulin sends the opposite message: insulin signals fat cells to slow or stop the release of fat. Since visceral fat cells are less responsive to insulin, having too many visceral fat cells leads to excess free fatty acid in the bloodstream, and the high level of free fatty acid eventually leads to hyperglycemia.
Hyperglycemia stimulates the pancreas to release more insulin. In this way, the excess free fatty acids have indirectly triggered, at least temporarily, higher-than-normal levels of circulating insulin (i.e., hyperinsulinemia).
If it had been subcutaneous fat cells that were releasing the excess fatty acids, the newly released insulin would “turn off the tap” by slowing or stopping the fatty acid release. Visceral fat cells, however, are less sensitive to insulin signals, and the feedback circuit is not very effective. When visceral fat is the source of excess free fatty acids, the natural balancing mechanisms do not work well, and hyperinsulinemia persists. This persistent hyperinsulinemia is a known contributor to insulin resistance (Levy & Nessen, 2022b).
FROM EXCESS FATTY ACIDS TO INSULIN RESISTANCE
- Persistent elevation of circulating free fatty acids causes hyperglycemia.
- Persistent hyperglycemia causes hyperinsulinemia.
- Persistent hyperinsulinemia causes insulin resistance.
This sequence of events shown in the box above can be expressed as the formula:
Fatty acids → Hyperglycemia → Hyperinsulinemia → Insulin resistance
The sequence can be triggered by anything that causes high blood levels of free fatty acids, glucose, or insulin. Conditions that lead to insulin resistance through this mechanism include high levels of glucocorticoids (e.g., Cushing’s disease or long-term treatment with prednisone), nonalcoholic fatty liver disease, and chronic elevated triglyceride levels.
OBESITY
Obesity has long been associated with a risk for type 2 diabetes. Risk factors for obesity include:
- Genetics. Genes may affect the amount of body fat a person has and where it is distributed. Genetics may also influence how efficiently the body converts food into energy and how the body burns calories during exercise.
- Family lifestyle. Family members generally share similar eating and activity behaviors.
- Inactivity. Without adequate exercise, people take in more calories than they burn, which can lead to weight gain.
- Unhealthy diet. Unhealthy diets can easily lead to obesity. Such diets are generally high in calories; lack adequate amounts of fruits and vegetables; and include fast foods, oversized portions, and high-calorie beverages.
- Medical conditions. Cushing’s syndrome and/or conditions that decrease activity, such as arthritis, can lead to weight gain.
- Medications. Some medications can lead to weight gain.
- Age. As one ages, hormonal changes and a less active lifestyle can contribute to weight gain.
- Lack of sleep. Getting too much or too little sleep can cause hormonal changes that increase appetite.
(Levy & Nessen, 2022a)
Because obesity puts a person at risk for type 2 diabetes, all the causes of obesity, from genes to lifestyle habits to medications, can contribute to a person’s tendency to develop type 2 diabetes (ADA, 2022a; Levy & Nessen, 2022a).
DRUGS THAT CAN CAUSE WEIGHT GAIN
- Antipsychotic drugs
- Antiseizure drugs
- Corticosteroids
- Traditional antidepressants (e.g., tricyclics, tetracyclics, monoamine oxidase inhibitors)
- Thiazolidinediones (e.g., rosiglitazone, pioglitazone)
- Beta blockers
- Benzodiazepines
(Levy & Nessen, 2022a)
IMMUNE SYSTEM ABNORMALITIES
There is now significant evidence to indicate that an overactive immune system response may actually target the beta cells of the pancreas, thus damaging these insulin-producing cells and adversely affecting insulin production. This phenomenon occurs mainly in patients with type 1 diabetes and may be an indication of an autoimmune cause of the disease (ElSayed et al., 2023a).
Recent clinical trials using hematopoietic stem cell therapy have shown benefits in the treatment of type 1 diabetes and could potentially be used to delay the onset of type 1 diabetes in high-risk individuals in the future (Pastore et al., 2021).
Abnormal Insulin Secretion
In addition to insulin resistance, people with type 2 diabetes have another key disorder. The beta cells in the pancreas of a person with type 2 diabetes do not secrete insulin normally. Together, insulin resistance and poorly functioning beta cells lead to the continual hyperglycemia that characterizes type 2 diabetes.
Insulin resistance means that a higher-than-normal amount of insulin in the bloodstream is needed to keep the plasma glucose levels at a normal level (<100 mg/dL). To maintain healthy blood glucose levels, the pancreatic beta cells in a person with insulin resistance are forced to secrete more than the normal amount of insulin. Therefore, people with insulin resistance generally have hyperinsulinemia.
Since people with type 2 diabetes usually have insulin resistance, they often have hyperinsulinemia. But even when they have hyperinsulinemia, the blood insulin levels are not high enough to prevent hyperglycemia. In other words, even when secreting high levels of insulin, their pancreas does not keep up with the demand. Part of the problem is that people with type 2 diabetes have fewer beta cells than normal. In addition, the existing beta cells in patients with type 2 diabetes do not secrete insulin as quickly and in as large amounts as normal.
Even before type 2 diabetes develops, beta cell problems can be detected in glucose tolerance tests, which give abnormal test results in prediabetic individuals. As with insulin resistance, beta cell dysfunction precedes the development of overt hyperglycemia by many years.
In another parallel with insulin resistance, treating type 2 diabetes can improve the functioning of the beta cells, but it cannot bring beta cell functioning up to normal. At present, both insulin resistance and beta cell dysfunction can be improved but not cured (Brutsaert, 2022).
Metabolic Syndrome
Metabolic syndrome is the name for a particular group of characteristics or health problems that are frequently found together. Metabolic syndrome is common in the United States, and over 40% of individuals age 50 years old or older have this condition. People with excess abdominal fat are more likely to have metabolic syndrome.
Core problems of metabolic syndrome are obesity and insulin resistance. Three additional problems are high blood pressure, high blood levels of triglycerides, and low blood levels of high-density lipoprotein (HDL) cholesterol. It is not clear whether metabolic syndrome causes type 2 diabetes, but it has been shown that having the syndrome increases a person’s chances of developing type 2 diabetes and cardiovascular disease (Levy & Nessen, 2022b).
DEFINITION OF METABOLIC SYNDROME
A diagnosis of metabolic syndrome is made if at least three of the following are present:
- Large waist circumference: a waistline that measures ≥35 inches (88 cm) for women and ≥40 inches (102 cm) for men
- Hypertriglyceridemia: ≥150 mg/dL (1.7 millimoles per liter)
- Low high-density lipoprotein (HDL) “good” cholesterol: ≤40 mg/dL in men or ≤50 mg/dL in women
- High blood pressure: ≥130/85 mmHg
- High fasting glucose: ≥100 mg/dL
(Levy & Nessen, 2022b)
CASE
George is a 50-year-old male being treated for hypertension. He arrives to the clinic for an annual physical. After stepping onto a scale, he is found to have gained 10 pounds over the previous year. His blood pressure has gradually been increasing over the past two years as well, with a current measurement of 140/88.
As his medical and family history is taken, George mentions that his mother and uncle were both diagnosed with diabetes after age 50. The nurse takes a measurement of his waist circumference, which is 105 cm (41 in).
After discussing the clinical picture with the primary care physician, a lipid panel is ordered. Three days later, the results of George’s blood test show blood triglycerides of 156 mg/dL and an HDL cholesterol level of 38 mg/dL.
George’s results indicate four criteria have been met for a diagnosis of metabolic syndrome. George is started on an antilipemic agent (a medication to treat hyperlipidemia), instructed on incorporating lifestyle interventions (e.g., diet, exercise), and given a referral to a dietitian at his request. A follow-up appointment is scheduled for three months later to assess how George is doing with initial management.
When George returns for his follow-up visit, he reports that he has been following his diet and exercise plan and feels that this has made a difference in how he is feeling. He has lost 8 pounds and 2 inches from his waist, his blood pressure is now 124/78, his triglycerides have improved to 130 mg/dL, and his HDL cholesterol has increased to 52 mg/dL.
George continues to be motivated to make changes in order to improve his health and states that he feels better than ever. He adds that his wife has been very supportive—together they are following a Mediterranean diet for meals and exercising on a regular basis.
SCREENING AND PREVENTION OF TYPE 2 DIABETES
Screening for Diabetes
Testing to detect type 2 diabetes and assess risk for future diabetes (prediabetes) in patients who are asymptomatic should be conducted for those who are overweight or obese and who have one or more additional risk factors for diabetes, such as:
- Physical inactivity
- First-degree relative with diabetes
- High-risk race/ethnicity (e.g., African American, Latino, Native American, Asian American, Pacific Islander)
- Hypertension (130/80 mmHg or higher, or receiving treatment for hypertension)
- HDL cholesterol level of 35 mg/dL or lower and/or a triglyceride level of 250 mg/dL or higher
- Individuals with polycystic ovarian syndrome
- History of cardiovascular disease
- Other clinical conditions associated with insulin resistance (e.g., severe obesity or acanthosis nigricans [skin condition characterized by areas of dark, velvety discoloration in body folds and creases])
The American Diabetes Association recommends screening for diabetes or prediabetes as follows:
- Every year in people with prediabetes (A1C of 5.7% or higher), impaired glucose tolerance (IGT), or impaired fasting glucose (IFG) on previous testing (see also “Laboratory Tests” later in this course)
- Every three years for individuals who were diagnosed with gestational diabetes (diabetes diagnosed during pregnancy that is not clearly overt diabetes)
- Every three years for individuals over the age of 35 and individuals with HIV infection
If results are normal, testing should be repeated at least at three-year intervals; more frequent testing should be considered depending on initial results and risk status (ElSayed et al., 2023a).
Prevention and Prediabetes
People who have abnormal carbohydrate metabolism but do not yet meet the criteria for a diabetes diagnosis have a condition called prediabetes, which places them at high risk of developing type 2 diabetes (ElSayed et al., 2023a). An estimated 96 million adult Americans have prediabetes, and most of them (81%) are unaware they have it (ADA, 2022a).
DEFINITION OF PREDIABETES
The diagnosis of prediabetes is made by a finding of either:
- Fasting plasma glucose = 100–125 mg/dL
or - Two-hour oral glucose tolerance test = 140–199 mg/dL
or - A1C = 5.7%–6.4%
(ElSayed et al., 2023a)
SCREENING FOR PREDIABETES
The American Diabetes Association recommends screening for prediabetes for all adults ages 35 and older every three years. Testing should also be completed for adults of any age who are overweight (defined as a body mass index [BMI] >25 kg/m2 or a BMI >23 kg/m2 for Asian American individuals) and have at least one of the additional risk factors for diabetes (as listed above) (ElSayed et al., 2023a).
Prediabetes can be recognized through the same screening tests used to diagnose diabetes. The simplest test is the fasting plasma glucose (FPG) level, defined as the plasma glucose level after eight hours or more without calorie intake. An FPG level is considered normal when it is less than 100 mg/dL. (The World Health Organization criteria sets the lower limit at 110 mg/dL.)
In prediabetes, FPG is in the impaired range (100–125 mg/dL) in measurements taken on two different days. This range is the diagnostic criteria for prediabetes set by the American Diabetes Association. Alternately, an oral glucose tolerance test in the impaired range (140–199 mg/dL at two hours), from two different test results, can be used to diagnose prediabetes (ElSayed et al., 2023a).
A fasting blood glucose reading of 126 mg/dL or higher on two separate tests is diagnostic of diabetes (ElSayed et al., 2023a).
In addition to signaling a person’s risk for developing type 2 diabetes, prediabetes warns that the person also has a higher risk for heart disease and stroke.
TREATING PREDIABETES
A program of weight loss and increased physical activity can improve the problems underlying prediabetes, and, many times, lifestyle changes alone can prevent people with prediabetes from going on to develop diabetes.
Recently, a task force of experts issued a set of guidelines for people diagnosed with prediabetes. These guidelines prompted the American Diabetes Association to recommend the same cardiovascular treatment goals for prediabetes as for diabetes. These goals include:
- HDL cholesterol levels >50 mg/dL for women and >40 mg/dL for men
- LDL cholesterol levels <70 mg/dL
- Blood pressure measured at every routine visit with healthcare providers
- Blood pressure ≤130/80 mmHg if this target can be safely achieved
(ElSayed et al., 2023d)
In terms of interventions when a person is diagnosed with prediabetes, the first step is to initiate lifestyle changes, including exercising and eating a healthy reduced-calorie diet (e.g., fruits, nonstarchy vegetables, lean meats, nonfat dairy products). (See also “Nutrition Management” later in this course.)
Weight-loss goals for people with prediabetes include losing at least 7% of their body weight and doing 150 minutes/week or more of moderate-intensity physical activity (such as brisk walking) (ElSayed et al., 2023c).
Individuals with prediabetes can greatly reduce their risk of progressing to diabetes by losing weight. In the Diabetes Prevention Program major randomized controlled trial, each kilogram an individual with prediabetes lost reduced their risk of disease progression by 16% over 3.2 years.
The antidiabetes drug metformin (Glucophage) is considered for use by high-risk patients with prediabetes who are unable to control their blood sugar with weight loss and exercise. Research shows that drugs such as metformin or thiazolidinediones (e.g., pioglitazone) can delay the onset of type 2 diabetes in specific populations (ElSayed et al., 2023c).
To date, there have been no pharmacologic agents approved by the U.S. Food and Drug Administration specifically to prevent type 2 diabetes. Medications that have been evaluated for weight loss and may be able to decrease the risk of diabetes progression in individuals with diabetes include:
- Orlistat
- Phentermine topiramate
- Liraglutide
- Semaglutide
- Tirzepatide
(ElSayed et al., 2023c)
Additionally, people with prediabetes should not smoke and should avoid excessive alcohol consumption (more than one drink a day for women and two drinks for men).
ASSESSMENT AND DIAGNOSIS OF TYPE 2 DIABETES
The health problems of diabetes are caused directly from hyperglycemia, and the medical diagnosis of the disease is not based on its cause but rather on evidence of persistent high plasma glucose levels, regardless of the cause. The American Diabetes Association recommends that diabetes be diagnosed based on the following criteria:
- A1C level of ≥6.5%* (A1C is recommended as a primary diagnostic test for diabetes.)
- Fasting plasma glucose level of ≥126 mg/dL*
- Two-hour plasma glucose level of ≥200 mg/dL in an oral glucose tolerance test (OGTT)*
- Random plasma glucose level of ≥200 mg/dL along with classic symptoms of hyperglycemia or hyperglycemic crisis
(ElSayed et al., 2023a) - * In the absence of unequivocal hyperglycemia, diagnosis requires two abnormal test results from the same sample or in two separate test samples.
Persons who do not know that they have diabetes may come to an office, clinic, or emergency department with hyperglycemia. Sometimes their hyperglycemia is discovered incidentally and with no other clues. On the other hand, these individuals may have symptoms of diabetes, such as polydipsia, polyuria, weakness, fatigue, blurred vision, headache, dizziness, or dehydration. At times, they already have diabetic complications (e.g., coronary artery disease, peripheral vascular problems, nonhealing wounds, or recurrent skin or genitourinary tract infections). Moderate to severe hyperglycemia in a person not previously known to have diabetes may also be triggered by another recent medical problem such as an acute infection or acute cardiac or kidney problems.
Laboratory Tests
An initial diabetes examination screens for abnormalities and also establishes baseline values that are used to evaluate the treatment program and to follow the progress of the disease objectively. Patients with diabetes may have frequent testing to assess the effectiveness of the treatment plan and measure changes in various lab values. Nurses and diabetes educators may be the primary point of contact for discussing the results of laboratory tests as a patient’s progress is tracked.
BLOOD GLUCOSE TESTS
Fasting Plasma Glucose (FPG)
Among the various measurements of the body’s ability to produce and use glucose, the blood level of glucose after an eight-hour fast is the standard. After eight or more hours without eating, the body should maintain plasma glucose levels in the range of 70–99 mg/dL. (Plasma glucose levels are also sometimes given in millimoles per liter, or mmol/l.)
People whose fasting (i.e., at least eight hours after eating) blood levels of glucose are slightly elevated (100–125 mg/dL) are not able to use glucose optimally and are considered to have prediabetes; they also have impaired fasting glucose (IFG). When a person’s FPG levels are ≥126 mg/dL, the person is said to have diabetes (ElSayed et al., 2023a).
| Category | Fasting Plasma Glucose Level |
|---|---|
| Normal | 70–99 mg/dL |
| Prediabetes | 100–125 mg/dL |
| Diabetes | ≥126 mg/dL |
Oral Glucose Tolerance Test (OGTT)
A more complicated test, the oral glucose tolerance test, can also be used to diagnose diabetes and gestational diabetes. In an OGTT, the patient drinks a sugar-water solution (75 g of glucose in water), and the plasma glucose level is measured after two hours. If the patient’s blood two-hour plasma glucose is ≥200 mg/dL, they meet the criteria for diagnosis of diabetes (ElSayed et al., 2023a).
A1C Test
The A1C test is also called the A1c, hemoglobin A1c, HbA1c, glycohemoglobin, glycated hemoglobin, and glycosylated hemoglobin test. This test is used to monitor a patient’s blood glucose levels during treatment and has been adopted by the American Diabetes Association as a recommended diagnostic test for diabetes (ElSayed et al., 2023a).
It is important for clinicians to understand A1C values and be able to explain what this level means to a patient who is diagnosed with diabetes. Overall monitoring of A1C levels is also important as an indicator for patients who are at increased risk for chronic complications of diabetes.
The A1C test measures the percentage of hemoglobin to which glucose molecules have become attached (i.e., the percentage of glycosylated hemoglobin). As a person’s plasma glucose level rises, more hemoglobin molecules become glycosylated, a condition wherein glucose sticks indiscriminately to proteins. The amount of glycosylated hemoglobin at any one time reflects the average plasma glucose level over the last three months (ElSayed et al., 2023a; Brutsaert, 2022).
The following chart shows the average plasma glucose levels that are indicated by various A1C values.
| Range | A1C Value | Estimated Average Blood Glucose (mg/dL) |
|---|---|---|
| (ADA, 2023a) | ||
| Alert Range | 10% | 240 |
| 9.5% | 226 | |
| 9% | 212 | |
| Elevated Range | 8.5% | 197 |
| 8% | 183 | |
| 7.5% | 169 | |
| Goal Range for Most Patients |
7% | 154 |
| 6.5% | 140 | |
| 6% | 126 | |
A 1% change in an A1C value reflects a change of about 30 mg/dL in average plasma glucose. Normal levels of plasma glucose produce an A1C value of about 5%. As the A1C value increases, so does the likelihood of complications.
In their May 2023 guidelines, the American Association of Clinical Endocrinologists (Samson et al., 2023) recommends that patients should aim for an A1C ≤6.5%. In other words, people with diabetes should try to keep their average blood glucose levels below 140 mg/dL. This has been shown to be a realistic goal and one that will improve the health of a wide variety of people with type 2 diabetes.
A less stringent A1C goals of <8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced renal disease, extensive comorbid conditions, or long-standing diabetes in whom the goal is difficult to achieve (Samson et al., 2023).
A1C values are averages, and A1C values will decrease (and therefore appear to be improved) if there are significant periods of excessively low plasma glucose levels (i.e., hypoglycemia). To ensure that they have not been artificially lowered by periods of hypoglycemia, blood glucose levels should be measured and recorded at key times (e.g., first thing in the morning, prior to meals, at bedtime, and sometimes two hours after meals). Also, A1C values will not reflect short swings in plasma glucose levels, as often happens when blood glucose levels are particularly labile.
To recognize hypoglycemic periods or short-term shifts in plasma glucose levels, patients should monitor their glucose levels regularly. The true level of glycemic control (using lifestyle changes and medications to avoid hyper- and hypoglycemia) can be seen best through a combination of A1C tests and daily blood glucose readings (Brutsaert, 2022; ElSayed et al., 2023a).
CASE
Sharon is a 46-year-old woman who presents to her primary care clinic reporting excessive urination over the last two months. An A1C test is ordered, and the results indicate a level of 6.8%. Additionally, her fasting plasma glucose is measured at 128 mg/dL, and her two-hour plasma glucose is 188 mg/dL. A repeat A1C test comes in at 7.2%, confirming a diagnosis of hyperglycemia and type 2 diabetes.
Sharon has a family history of diabetes as well as high blood pressure. The nurse meets with Sharon to provide initial education, support, and resources. The nurse reviews the importance of monitoring A1C levels and explains to Sharon that this test gives the best idea of overall glucose control. The nurse stresses that the goal for Sharon’s A1C level is less than 7.0%. The nurse reviews Sharon’s personal goals for daily blood sugar checks as well as A1C testing every three months.
BLOOD LIPID TESTS
A lipid profile is ordered when a patient is diagnosed with type 2 diabetes in order to evaluate for dyslipidemia (an unhealthy level of blood lipids). Dyslipidemia increases a person’s risk of developing a variety of health problems, most notably atherosclerotic cardiovascular disease. Dyslipidemia can include:
- Elevated blood levels of triglycerides
- Reduced blood levels of high-density lipoprotein (HDL)
- LDL particles that are smaller and denser than usual and contain more than the normal amounts of free cholesterol (which means that the cholesterol in type 2 diabetes is more easily added to atherosclerotic plaque)
| Lipids | Levels (mg/dL) |
|---|---|
| Triglycerides |
|
| HDL cholesterol |
|
| LDL cholesterol |
|
The dyslipidemia of type 2 diabetes is not always improved by simply reducing the patient’s hyperglycemia; the dyslipidemia may require direct treatment with statins or other lipid-lowering therapies (ElSayed et al., 2023d).
LIVER ENZYMES
Individuals diagnosed with type 2 diabetes also receive baseline liver function tests. Complications of diabetes that involve the liver include nonalcoholic steatohepatitis, liver fibrosis, and nonalcoholic fatty liver disease. Liver function tests resulting in high levels of alanine transaminase (ALT) and aspartate transaminase (AST) may indicate that the liver is not functioning properly. Since many antidiabetic medications are deactivated in the liver, the treatment plan may need to be adjusted (ElSayed et al., 2023e).
URINE TESTS
At one time, diabetes treatment was monitored by measuring the amount of glucose in the urine. Finger-stick blood glucose measurements are more sensitive and more accurate, and they have replaced urine tests for monitoring daily plasma glucose levels.
Urine Glucose Levels
In the kidneys, glucose that is initially filtered from the blood is almost fully reabsorbed before the urine is excreted. This reabsorption is very efficient, even when there is an excess of blood glucose up to levels of about 180 mg/dL. (Reabsorption is not absolute; normal urine does contain a small amount of glucose.)
By the time measurable sugar appears in the urine, hyperglycemia is already at an unhealthy level. Nonetheless, urine testing is an easy and quick warning of mild hyperglycemia, and urine tests are sometimes used for screening. Commercial plastic or paper strips (e.g., Clinistix, Diastix, Multistix, Uristix) can be dipped in fresh urine and will change color based on the different concentrations of sugar.
Urine Ketone Levels
Under normal conditions, ketones are not found in urine. Testing urine for ketone levels can determine whether fatty acids are being used for energy instead of glucose. In persons with diabetes that is poorly controlled, massive fatty acid catabolism can occur. This catabolism is the body’s attempt to provide an energy source when glucose cannot be transferred into the cells. The presence of ketones in the urine can be a sign of diabetic ketoacidosis (DKA), which is considered a medical emergency (CDC, 2022c). (See also “Diabetic Ketoacidosis” later in this course.)
Urine Albumin Levels
Protein (albumin) leaking into the urine of a person with diabetes usually indicates kidney damage. Albuminuria (excess albumin in the urine) can be detected through spot or 24-hour urine testing, which is recommended once per year for patients with type 2 diabetes (Brutsaert, 2022).
RENAL FUNCTION TESTS
Diabetes is the leading cause of end-stage renal disease, and an estimated 20%–40% of patients with diabetes have chronic kidney disease. The American Diabetes Association recommends monitoring indicators of kidney function at least once a year for patients with diabetes. Urinary albumin (e.g., spot urine albumin-creatinine ratio [uACR]) and estimated glomerular filtration rate (eGFR) are checked in all people with type 2 diabetes regardless of treatment. Persons with urinary albumin >300 mg/g Cr and/or an eGFR 30–60 mL/min/1.73 m2 should be monitored twice annually to guide therapy.
Patient Examination
A patient examination and assessment is a team effort and may include a medical examination, a nursing assessment, and input from specialty care providers to rule out and diagnose any comorbid conditions that are related to diabetes. The goal of an initial evaluation is to understand the health of the individual from head to toe. For a person who has or is suspected of having diabetes, there are five specific objectives (ElSayed et al., 2023e):
- Confirm the diagnosis and classify the diabetes
- Detect diabetes complications and potential comorbid conditions
- Review previous treatment and risk factor control in patients with established diabetes
- Begin patient engagement in the formulation of a care management plan
- Develop a plan for continuing care
(ADA, 2022c)
MEDICAL EVALUATION
The key elements of a comprehensive diabetes medical evaluation at the diagnostic visit and follow-up visits include assessment of the following:
- Past medical history, including age and onset of symptoms of diabetes
- Family history of diabetes
- History of complications, including comorbidities and hypertension
- Behavior factors, including eating patterns and weight history
- Current medication plan and medication-taking behavior
- Technology use, including use of health apps, glucose monitoring, or insulin pump
- Social life assessment, such as existing social supports
(ElSayed et al., 2023e)
LIFESTYLE AND SOCIAL HISTORY
On the basis of creating a plan of care for the person with suspected type 2 diabetes, the clinician collects information about lifestyle and social history. It is also important to collect baseline information about dietary habits; therefore, an important component is to have individuals write down their typical daily diet (ElSayed et al., 2023e).
A practical approach for assessing educational needs is also an important consideration for healthcare professionals. The following questions may assist in collecting baseline educational needs:
Diet Habits
- What do you eat for breakfast, lunch, and dinner?
- Do you have snacks between breakfast and lunch, lunch and dinner, dinner and bedtime? If so, what do you eat?
- What do you drink during the day?
Lifestyle and Social Habits
- How much exercise do you get each week?
- Do you smoke or have you ever smoked?
- Do you have social support from friends and family?
Educational Needs
- How do you prefer to learn new information?
- What information and resources have you already consulted?
- What do you know about diabetes and diabetes self-care?
- Do you have family or others who will be involved in your care?
PHYSICAL EXAM
Many people with type 2 diabetes already have complications present at the time of diagnosis. Therefore, the physical exam at the initial visit includes an assessment for signs and symptoms of diabetic complications and other problems—such as abdominal obesity or hypertension—that may compound the risks posed by diabetes. (See also “Hyperglycemia-Related Illnesses and Complications” later in this course.)
Primary components of a physical exam include the following assessments:
- Height, weight, and BMI measurements
- Blood pressure, including orthostatic measurements
- Thyroid palpation
- Skin examination (assess for acanthosis nigricans, autonomic neuropathy, diabetic ulcers, and skin erosions)
- Comprehensive foot examination (pulses, reflexes, sensation)
- Screening for depression, anxiety, and disordered eating
(ElSayed et al., 2023e)
In addition to a physical exam by their primary care physician, persons newly diagnosed with type 2 diabetes are referred for initial and ongoing visits with the following specialty clinicians:
- Eye care professional such as an optometrist to conduct annual dilated eye exams to screen for retinopathies, cataracts, and glaucoma
- Family planning for individuals of childbearing potential
- Dentist for regular dental exams and screening for periodontal (gum) disease
- Cardiologist to screen for coronary artery disease (CAD) and cardiovascular disease (CVD)
- Podiatrist for individuals experiencing reduced microvascular and macrovascular circulation, poor healing, and peripheral neuropathy (damage to the nerves outside of the brain and spinal cord)
- Audiologist, if indicated
- Mental health professional, if indicated
- Social worker, if indicated
(ElSayed et al., 2023e)
(See also “Hyperglycemia-Related Illnesses and Complications” later in this course.)
Obesity and BMI
The most commonly used measure of obesity is the body mass index (BMI). BMI has been shown to be a good indirect indication of the percentage of body fat, and it is the most commonly used measure of total body fat. It is measured using the formula:
BMI = weight in kilograms ÷ height in meters squared
or
BMI = 703 × (weight in pounds ÷ height in inches squared)
(CDC, 2022b)
The BMI obesity definitions for adults are shown in the table below.
| Definition | BMI | |
|---|---|---|
| (NHLBI, n.d.; Levy & Nessen, 2022a) | ||
| Normal | 18.5–24.9 | |
| Overweight | 25.0–29.9 | |
| Obese | Class 1 | 30.0–34.9 |
| Class 2 | 35.0–39.9 | |
| Class 3 (extreme obesity) | >40.0 | |
The American Diabetes Association recommends testing for diabetes in all adults who are overweight (BMI >25 kg/m2) and have one or more additional risk factors for diabetes (ElSayed et al., 2023a).
Blood Pressure
Blood pressure is to be measured at every routine clinical visit. Patients found to have elevated blood pressure (≥130/80 mmHg) should have blood pressure confirmed using multiple readings, including measurements on a separate day, to diagnose hypertension. In a meta-analysis that included 73,913 participants with diabetes, results showed that intensive blood pressure control lowered the risk of stroke by 31% (ElSayed et al., 2023d).
According to the American Diabetes Association (ElSayed et al., 2023d), a shared decision-making process should be used by patients and clinicians when setting individual blood pressure targets. Potential adverse effects of antihypertensive therapy (e.g., hypotension, syncope, falls, acute kidney injury, and electrolyte abnormalities) are a possibility and vary among patients. Persons with older age, chronic kidney disease, and frailty can be particularly vulnerable to adverse effects of strict blood pressure control.
CASE
Carol is a 52-year-old White woman with no previous history of diabetes who presents to the clinic with mild hyperglycemia (290 mg/dL), low HDL cholesterol (33 mg/dL), and microalbuminuria. Carol appears to be overweight, and the nurse calculates her BMI to be 29 kg/m2. She also reports recurrent urinary tract infections (previous infections twice in the past four months).
The nurse continues the assessment by asking Carol about any classic symptoms or complications of diabetes, such as weakness, fatigue, blurred vision, headache, dizziness, or dehydration. The nurse also asks Carol about her family history of diabetes and discovers that her mother has been diagnosed with type 2 diabetes.
Based on her assessment of Carol, the nurse suspects diabetes. The nurse discusses Carol’s case with the primary care physician, who orders A1C, fasting plasma glucose, and two-hour plasma glucose labs for Carol.
DEVELOPING A TYPE 2 DIABETES TREATMENT PLAN
When developing a type 2 diabetes treatment plan, it is important to begin by establishing the patient’s goals, setting up a care management team, and understanding the needs of someone with newly diagnosed type 2 diabetes.
Goals of Treatment
The primary goals for the treatment plan of patients with type 2 diabetes, according to the American Diabetes Association, are to prevent complications and optimize quality of life.
A shared decision-making process is recommended when creating the treatment plan and includes motivational interviewing and goal setting. The following individual preferences, values, and goals are assessed during development of the treatment plan:
- Age
- Cognitive abilities
- School/work schedule and conditions
- Health beliefs
- Support systems
- Eating patterns
- Physical activity
- Social situation
- Financial concerns
- Cultural factors
- Literacy and understanding of math
- Diabetes history
- Comorbidities
- Disabilities
- Health priorities
- Other medical conditions
- Preferences for care
- Life expectancy
(ElSayed et al., 2023e)
INDIVIDUALS WITH TYPE 2 DIABETES WHO ARE PREGNANT
Individuals diagnosed with type 2 diabetes who become pregnant pose special challenges and therefore require special care. (It is important to note that there is a difference between a person who has type 2 diabetes who becomes pregnant, a person who becomes pregnant and is found to have undiagnosed type 2 diabetes, and a person who becomes pregnant and is diagnosed with gestational diabetes.)
The obesity and diabetes epidemic has resulted in more people of reproductive age having type 2 diabetes and likely not knowing they have it. Early screening before 15 weeks’ gestation can help to identify individuals who have undiagnosed type 2 diabetes. Individuals who are negative for type 2 diabetes in early screening are to be screened for gestational diabetes between 24 and 28 weeks of gestation (ElSayed et al., 2023a).
During pregnancy, weight-loss programs should be terminated, oral hypoglycemic medications may be contraindicated, and insulin therapy should be intensified. Congenital malformations are more common in pregnancies when diabetes is not well controlled, and infants are often of larger-than-normal birth weight. These and other potential complications make it important for individuals of reproductive age with diabetes to understand the need for glucose control. Their diabetes care teams should include nurse-midwives or obstetricians specializing in diabetes.
Care Management Team
Ideally, persons with type 2 diabetes are treated by a multidisciplinary team of healthcare professionals working together. The many necessary interactions with team members, especially at the beginning of therapy, are coordinated based on each person’s individual needs (Davis et al., 2022).
- Primary care provider or endocrinologist: Leads the team in the care and management of the patient with type 2 diabetes; coordinates the initial diagnosis and medical recommendations for treatment
- Registered nurses: Work closely with the person with diabetes, their family, and other team members to educate and support the individual and family as the plan of care and treatment are initiated; provide continuing nursing support through ongoing monitoring
- Dietitians: Work closely with the patient and family to assist in educating and supporting the patient about dietary recommendations, including any special diets for weight reduction and later maintenance
- Ophthalmologists: Provide specialty examinations focused on eye health, including annual fundoscopic, dilated-eye assessments
- Podiatrists: Provide regular support and specialty care with assessment, evaluation, and management of foot care, including prevention and treatment strategies
- Dentists and registered dental hygienists: Work closely with the patient to provide regular cleaning and hygiene, screening exams for gum and tissue changes, and treatment for dental cavities
- Pharmacists: Provide support and education on how to organize and administer diabetes medications, recognize precautions or interactions with other medications, and note any side effects and long-term effects of the patient’s medication regimen
- Physical therapists: Evaluate and create a plan to address any physical rehabilitation, functional mobility, and therapeutic exercise/activity needs, with ongoing monitoring of progress; recommend and fit assistive devices to assist the patient with ambulation or other forms of mobility as needed
- Occupational therapists: Evaluate patients for low vision, cognitive impairment, and fine motor skills and create a plan to address the patient’s activities of daily living and assess for and recommend home health care needs, referral to social services, and assistive devices
- Exercise physiologists: Create and monitor the patient’s plan for initiating a formal exercise plan, which may include goals for weight loss and healthy exercise habits
- Psychological counselors: Address and provide support for the emotional and psychological impact of a diagnosis of diabetes, including an increased risk for depression and social isolation
- Certified diabetes care and education specialists (CDCES): Provide education, direct care, and self-management interventions for patients with diabetes and their families (see also “Diabetes Educators” later in this course)
Individuals with diabetic medical complications may be referred to specialty providers such as ophthalmologists, cardiologists, renal specialists, podiatrists, psychiatrists, and prosthetists. The team of health professionals caring for a person with diabetes should take a holistic approach to caring for their client’s health (ElSayed et al., 2023f).
ROLE OF PHYSICAL THERAPY
Physical therapists may assist patients with type 2 diabetes through the following:
- Creating safe, individualized exercise programs to improve functional mobility, reduce pain, and improve blood glucose levels
- Assessing strength, flexibility, endurance, gait, and balance (static and dynamic)
- Helping prepare a patient for the functional mobility-related aspects of surgery and recovery
- Teaching patients how to use assistive devices and/or a prosthesis
- Assisting patients to heal and/or manage circulation and skin problems
(APTA, 2020)
Reviewing a Patient’s Glycemic Control
An important aspect of a type 2 diabetes treatment plan is establishing blood glucose targets and monitoring blood glucose levels to determine whether the patient’s blood glucose levels are being properly controlled with the current interventions. Two sets of data are used to review a patient’s glycemic control: A1C values and daily blood glucose records.
The A1C values show the average level of hyperglycemia in the preceding three months (see table earlier in this course to translate A1C values into average blood glucose levels). The target for adults with diabetes is usually an A1C of <7%, or about 154 mg/dL average blood glucose level. Although the ideal would be an A1C of <7%, it is difficult for most people with diabetes to reach these low A1C values without having significant periods of hypoglycemia. With this in mind, less stringent goals are set for some individuals (ElSayed et al., 2023h; Samson et al., 2023).
In addition to A1C values, the patient’s daily glucose levels are reviewed regularly. Patients whose blood glucose values are close to their targets are reexamined every six months. Patients whose blood glucose values are out of the target range or whose medications have changed are reexamined every three months.
People with type 2 diabetes are at risk for developing cardiovascular disease; therefore, blood pressure and lipid profiles are monitored. Over time, elevated blood glucose can damage cardiovascular blood vessels, which in turn impedes cardiovascular blood flow and increases the risk for cardiovascular disease. Target goals for blood pressure and cholesterol are:
- Blood pressure <130/80 mmHg (less stringent goals may be set for some individuals)
- Fasting plasma HDL cholesterol >40 mg/dL in men, >50 mg/dL in women
- Fasting plasma triglycerides <150 mg/dL
(ElSayed et al., 2023d)
USING SENSITIVE LANGUAGE
Language is important, and how a person says something can affect how they see themselves and their future actions. A clinician’s language can either empower the patient or make them feel powerless. The American Diabetes Association (ElSayed et al., 2023e) has detailed five key recommendations regarding language use for healthcare professionals to use when communicating with people with type 2 diabetes:
- Use neutral language that is not judgmental and is based on facts and actions.
- Use stigma-free language.
- Use respectful language that is strength based, inclusive, and gives the patient hope.
- Use language intended to encourage collaboration between the person with diabetes and their healthcare team.
- Use person-centered language, such as “person with diabetes” instead of “diabetic.”
LIFESTYLE CHANGES AND SELF MANANGEMENT
The overall treatment plan for a person with diabetes includes a patient education program. The patient is an integral member of the treatment team and must understand and be involved in developing their particular plan.
The education and involvement of patients with type 2 diabetes is an ongoing process. Patients receive continual education about primary lifestyle changes used to manage and treat type 2 diabetes, including nutrition management, increased physical activity, smoking cessation, and weight management. Lifestyle interventions have the ability to reduce hyperglycemia and reduce A1C in a person with type 2 diabetes. Lifestyle changes may also improve many of the health problems that often accompany type 2 diabetes, notably obesity, hypertension, and dyslipidemia (ElSayed et al., 2023f).
In addition, thorough education for patients on insulin therapy includes how to monitor blood glucose plus symptoms of and interventions for hypoglycemia.
All individuals with diabetes should be offered diabetes self-management education and support (DSMES) services. The goals of DSMES include:
- Encouraging informed decision-making
- Improving self-care behaviors
- Problem-solving
- Active collaboration with the healthcare team
DSMES services empower the patient with tools to optimize their self-care. The individual’s confidence level, health literacy, and burden of treatment should be assessed at the initiation of DSMES services (Davis et al., 2022; ElSayed et al., 2023f).
DIABETES EDUCATORS
Patient education is an entire program of its own, with trained educators who meet with the patient regularly and who are available for questions between visits (ElSayed et al., 2023f; Meneghini, 2020). The diabetes nurse educator is an important member of the healthcare team. Additionally, the Association of Diabetes Care and Education Specialists (ADCES) provides the names of local diabetes educators and contact information for education programs throughout the country.
DSMES services should be patient centered and may be provided in group or individual settings and/or use technology for support. Good communication with the entire diabetes care team is key. Diabetes self-management may be coordinated by one or more trained professionals with specialty certification in diabetes.
Diabetes educators are specialty educated and licensed and may include registered nurses, registered dietitians, pharmacists, or other specialists. Diabetes educators have the opportunity to earn two different credentials: Certified Diabetes Care and Education Specialist (CDCES) or Board Certified-Advanced Diabetes Management (BC-ADM). The BC-ADM credential is for advanced-level practitioners (Davis et al., 2022; ElSayed et al., 2023f).
Topics in DSMES education include the seven ADCES self-care behaviors that provide an evidence-based base that can be used as an adjunct to a diabetes education program. The seven ADCES Self-Care Behaviors are:
- Healthy coping with psychosocial issues and concerns
- Healthy eating
- Physical activity
- Medication usage
- Problem-solving
- Monitoring and using patient-generated health data
- Reducing risks
(ADCES, 2023; Davis et al., 2022)
The ADCES Self-Care Behaviors are recommended by the American Diabetes Association to be used toward monitoring the progress of individualized goals (Davis et al., 2022).
Healthy Coping With Psychological Issues and Concerns
Patients with diabetes face multiple lifestyle and behavioral demands, including medication dosing and titration, monitoring blood glucose, food intake, and physical activity. This constant pressure to manage their condition may cause increasing stress as time goes on, whether or not the disease progresses.
Diabetes distress is therefore a common and distinct concern for patients with diabetes. The American Diabetes Association defines diabetes distress as “significant negative psychological reactions related to emotional burdens and worries specific to an individual’s experience in having to manage a severe, complicated, and demanding chronic condition such as diabetes.”
Approximately 18%–45% of patients with type 2 diabetes report experiencing diabetes distress. There is a positive correlation between high levels of diabetes distress and higher A1C levels. Diabetes distress can also lead to suboptimal eating and exercise behaviors, feelings of anxiety and depression, and lower health-related quality of life.
Patients should be screened for diabetes distress using validated measures. If diabetes distress is identified, the clinician acknowledges it and provides an appropriate referral for additional support (e.g., social, emotional, and financial). Education may be recommended as well as a referral to a mental health provider for assessment and management (ElSayed et al., 2023f).
CASE
Diego is a 40-year-old Hispanic male with a recent diagnosis of type 2 diabetes. The nurse, Juana, is conducting a three-month follow-up visit to Diego’s initial diagnostic appointment. During the appointment, Diego is very quiet and speaks only when the nurse asks him direct questions. Juana uses nonjudgmental language that is respectful and imparts hope.
Juana says, “Diego, last time we discussed making some changes to how you eat and increasing your movement each day. I understand that change can be very difficult. Would you like to talk more about how we can help you improve your health and prevent complications?” She notices that Diego is looking at his feet and avoiding eye contact when she speaks.
Diego responds, stating that he has been feeling very overwhelmed since his diabetes diagnosis. He tells Juana that his father died when he was only 62 years old from diabetes complications and that he is afraid he will end up dying at a young age, like his father. She recognizes the symptoms of diabetes distress. Using patient-centered, sensitive language, Juana communicates to Diego that she recognizes that it sounds like he’s experiencing a lot of stress and anxiety about his diagnosis.
The nurse confers with the medical provider, who refers Diego to a mental health provider for an assessment. Diego is able to start cognitive behavioral therapy and reports at the next appointment that he is feeling more empowered to manage his diabetes with lifestyle modifications and medication therapy.
Healthy Eating
Nutrition therapy is an important aspect of lifestyle modifications for people with type 2 diabetes. The American Diabetes Association recommends that meal planning be individualized, since there cannot be a “one-size-fits-all” approach. There is no exact mix of nutrients that comprises the optimal diet for people with type 2 diabetes (ElSayed et al., 2023f).
Patients are given a referral to a registered dietitian who is knowledgeable and skilled in medical nutrition therapy specific to diabetes when they are first diagnosed. Consultation with a dietitian continues regularly throughout the course of their treatment. Research shows that patients who receive nutrition management services from a registered dietitian have a resulting decrease of 0.3%–2.0% in their A1C level.
The American Diabetes Association recommends that nutrition therapy for persons with type 2 diabetes be an ongoing process throughout management of their condition. To achieve weight loss and prevent excess weight gain, a multifaceted approach is needed, including nutrition interventions, lifestyle changes, and ongoing support (ElSayed et al., 2023f).
The goals of nutrition therapy for adults with diabetes include:
- Promoting and supporting healthful eating patterns, including the promotion of nutrient-dense foods to be eaten in properly portioned sizes
- Addressing the nutritional needs of an individual, taking into consideration their personal and cultural preferences, health literacy, access to healthy foods, willingness to change, and barriers to change
- Providing nonjudgmental feedback about food choices and limiting food choices when backed by evidence
- Providing the patient with tools that they can use to create healthy sustained eating patterns versus focusing on single foods
(ElSayed et al., 2023f)
Examples of healthy eating that have shown positive results in research include the following:
- Mediterranean eating pattern
- Dietary Approaches to Stop Hypertension (DASH) eating pattern
- Diabetes plate method
- Low-carbohydrate eating pattern
- Vegetarian/plant-based eating patterns
- Reduced saturated and trans fat eating patterns
- Carbohydrate-counting eating pattern
(ElSayed et al., 2023d)
However, it is important to note that not one eating pattern has consistently been shown to be the best plan for people with type 2 diabetes (ElSayed et al., 2023f).
DIABETES PLATE METHOD
The diabetes plate method is often taught to individuals managing type 2 diabetes. This method provides a visual way to plan a meal of healthy vegetables, lean proteins, and limited higher carbohydrate foods that can cause a spike in blood glucose.

Diabetes plate method. (Source: CDC.gov)
Start with a 9-inch dinner plate:
- Fill half with nonstarchy vegetables, such as salad, green beans, broccoli, cauliflower, cabbage, or carrots.
- Fill one quarter with a lean protein, such as chicken, turkey, beans, fish, or eggs.
- Fill one quarter with a grain or starchy food, such as potatoes, rice, or pasta.
(ADA, 2023b)
HOW TO READ FOOD LABELS
The Nutrition Facts label is required by the U.S. Food and Drug Administration (FDA) on most packaged foods and beverages. The label provides detailed information about a food’s nutrient content, such as the total amount of calories, serving size, amount and kinds of fat, added sugar, sodium, fiber, and other nutrients. Knowing how to read food labels is especially important for patients with diabetes who need to follow a special diet. The label also makes it easier to compare similar foods to decide which is a healthier choice.
(Source: U.S. Food and Drug Administration.)
The following tips can help patients with diabetes read and understand food labels:
- Start by reading the serving size and the number of servings in the package. Serving sizes are standard, making it easier to compare similar foods. Serving sizes are usually listed in common terms, such as cups or pieces, as well as in metric amount (e.g., grams).
- Next review the calories. This section provides information on how much energy (in calories) is provided in the food.
- The following section of the label contains information about specific nutrients. Nutrients listed in the first section are those that may need to be limited (e.g., fat, saturated fat, trans fat, cholesterol, or sodium). Listed next, total carbohydrates are a key component, and patients should understand their goals for total carbohydrates for each meal and decide the portion size to match. Nutrients listed in the last part of this section are important to include in a balanced diet (e.g., dietary fiber, vitamin A, vitamin C, calcium, and iron).
- The right-hand column and footnote area of the label provide information on Daily Values (DVs) for each nutrient listed and are based on public health experts’ advice. DVs are recommended levels of intake. DVs in the footnote are based on a 2,000-calorie diet.
(U.S. FDA, 2022)
MEDITERRANEAN EATING PLAN
Results from randomized controlled trials show that a Mediterranean-style eating pattern can improve both glycemic control and blood lipids. This style of eating is how inhabitants of countries bordering the Mediterranean Sea typically eat. Highlights of a Mediterranean eating plan include:
- Eating primarily plant-based foods
- Replacing butter with olive oil as the primary source of fat
- Using herbs and spices instead of salt
- Limiting red meat consumption to no more than a few times a month
- Eating fish and poultry at least twice a week
(ElSayed et al., 2023d, 2023f)
CARBOHYDRATE COUNTING
Carbohydrate counting (also called carb counting) is a more advanced method than the diabetes plate method that has been shown to improve A1C. In the carb-counting method, the amount of carbohydrates that are eaten at each meal are calculated and added up. Carbohydrates can be estimated by summing the approximate grams in each serving. The labels of most foods help patients to make these estimates (see “How to Read Food Labels” above) (ADA, 2023c).
This method requires a higher level of health literacy, more time, and greater commitment. Most individuals use the carb-counting method when they are taking mealtime insulin. These individuals should receive intensive and continual education regarding how to calculate the carbohydrate amount and apply it to determine the required insulin dosage (ElSayed et al., 2023f).
Carbohydrate counting when an individual uses mealtime insulin may be done using the following method:
- The person has an insulin-to-carbohydrate ratio (ICR) in which they count the carbohydrates they are going to consume.
- They then calculate the amount of insulin to take based on the ICR.
(ADA, 2023c)
Many bedside nurses are familiar with the carb-counting method, as it is used regularly for hospitalized patients with diabetes who are on insulin. When caring for these patients, providers can educate them at each mealtime by calculating the number of carbs together with the patient and explaining the dose of insulin to which it corresponds.
CASE
Deepa is a bedside nurse who is caring for Diane, a 57-year-old female. The hospitalist team is expecting that Diane will be discharged tomorrow. Diane has a secondary diagnosis of type 2 diabetes and will be starting mealtime insulin after she is discharged to help control her diabetes. The hospitalist has ordered sliding-scale insulin doses to be given with breakfast, lunch, and dinner while Diane is in the hospital.
Diane is about to eat her breakfast. Deepa checks Diane’s blood glucose level, which is 120. Deepa explains to the patient that she needs to calculate how many carbohydrates are in Diane’s breakfast so she can administer the appropriate corresponding dose of insulin. Diane is eating one serving of scrambled eggs, one small blueberry muffin, one cup of mixed fruit, and coffee with one creamer pod. Deepa calculates the number of carbs using the ticket that has come with the tray. The ticket shows:
- Scrambled eggs = 1 carb
- Blueberry muffin = 75 carbs
- Cup of mixed fruit = 30 carbs
- Coffee = 0 carbs
- Creamer pod = 2 carbs
Deepa educates Diane on the amount of carbohydrates she is eating, which she calculates to be 108 grams. The insulin dose ordered is 5 units of fast-acting insulin for carbohydrate consumption between 100 and 150 grams. Deepa explains how she added up the carbohydrates and how Diane can use food labels to estimate the carbohydrate content of foods at home. Deepa confirms that Diane intends to eat the whole meal and administers the 5 units of insulin after Diane begins to eat.
MEDICAL NUTRITION THERAPY RECOMMENDATIONS
Studies show that there is not a set percentage of carbohydrates, proteins, and fats recommended for people with type 2 diabetes. Care is individualized according to the patient’s goals, preferences, and normal patterns of eating. Different types of macronutrients (carbohydrates, proteins, and fats) are assessed in such an individualized eating plan, together with micronutrients, non-nutritive sweeteners, and alcohol (ElSayed et al., 2023f).
Carbohydrates
Studies have not yet determined the perfect amount of carbohydrates a person with diabetes should eat. However, the evidence is clear that blood glucose can be better controlled with calculated carbohydrate consumption that takes into consideration how the body metabolizes carbohydrates.
Patients are encouraged to consume high-quality, nutrient-dense carbohydrates that have a lot of fiber and are not highly processed. Fiber increases the amount and diversity of “good” bacteria in the gut, and high-fiber carbohydrate sources such as vegetables, whole grains, and legumes are encouraged. The recommended intake of fiber is at least 14 grams per 1,000 calories consumed (ElSayed et al., 2023f).
| Recommended | Not Recommended |
|---|---|
| (ADA, 2023c) | |
|
|
Protein
There is not a consensus on the ideal amount of protein that individuals with diabetes should consume. Currently, the recommendation is that protein consumption be individualized according to the patient’s eating habits and following the advice of a registered dietitian for the specific individual. A higher level of protein is considered 20%–30% of the food consumed, and results from studies show that this amount of protein may improve blood glucose levels by improving the feeling of fullness and satisfaction after eating, which may lead to lower consumption levels (ElSayed et al., 2023f).
The daily recommended allowance for protein is 0.8 grams per kilogram (0.36 grams per pound). A minimum of this amount should be consumed to improve glycemic measures, minimize cardiovascular risk, and minimize malnutrition risk (ElSayed et al., 2023f). For a 200-pound individual, this would mean consuming at least 72 grams of protein per day.
Fats
For individuals with type 2 diabetes, the type of fat that they eat is more important than how much total fat they consume. Saturated fat consumption should be limited to reduce cardiovascular risk and improve metabolic goals. The following foods are high in saturated fats and should be limited:
- Processed meats such as bacon and sausage
- Chicken or turkey skin
- Egg yolks
- Butter
- Lard and shortening
- Hydrogenated and partially hydrogenated vegetable oils
- High-fat dairy products such as cream
- Baked goods made with butter, such as cookies, cakes, muffins, and pastries
People with diabetes should be encouraged to limit their consumption of saturated fat and dietary cholesterol according to the recommended daily amounts for the general population. In addition, they should avoid consuming trans fats. Patients are also advised not to replace saturated fats with refined carbohydrates (ElSayed et al., 2023f).
| Recommended | Not Recommended |
|---|---|
|
|
Micronutrients
For patients without specific deficiencies, the American Diabetes Association does not have a clear recommendation for vitamin or mineral supplementation. Results from studies have not shown a clear benefit for whether or not an individual with type 2 diabetes should take a vitamin.
Individuals with type 2 diabetes who are taking metformin could have a vitamin B12 deficiency and should be tested periodically. Individuals who are older adults, pregnant, lactating, vegetarians, or following low-carbohydrate or low-calorie diets may need to take a multivitamin, but care should be individualized according to their specific needs (ElSayed et al., 2023f).
Non-nutritive Sweeteners
Non-nutritive sweeteners (sweeteners that have few or no calories) have not been found to have a significant impact on blood glucose levels. Additionally, data from some studies indicate that non-nutritive sweeteners can be beneficial for weight loss, but other studies report an association with weight gain. The American Diabetes Association states that individuals with a “sweet tooth” may be advised to use non-nutritive sweeteners in moderation in order to not feel deprived (ElSayed et al., 2023f).
Alcohol
Water as the primary source of hydration is always encouraged for consumption by individuals with type 2 diabetes. Moderate alcohol consumption has not been found to have a significant health impact on individuals with type 2 diabetes. Alcohol consumed in excess can increase the risk of hypoglycemia, weight gain, and hyperglycemia. Individuals with type 2 diabetes who choose to drink alcohol should be advised to monitor their blood glucose frequently to reduce risk of these adverse events.
All individuals with type 2 diabetes are advised to follow current alcohol consumption guidelines, which is no more than one drink per day for women and no more than two drinks per day for men. (One drink is a 12-ounce beer, a 5-ounce glass of wine, or 1.5 ounces of hard alcohol.) (ElSayed et al., 2023f).
Physical Activity
An integral component of lifestyle modification for people with type 2 diabetes is increased physical activity. Physical activity is a broad term that encompasses all movement that improves energy use and minimizes sedentary activity. Exercise is defined by the American Diabetes Association as “a more specific type of physical activity that is structured and designed to improve physical fitness.” Physical activity and exercise are both important components of a diabetes management plan.
The many proven benefits of exercise include:
- Improved blood glucose levels
- Reduction of cardiovascular risk factors
- Weight management benefits
- Improved well-being
The American Diabetes Association recommendations include:
- Children and adolescents with diabetes or prediabetes to engage in 60 minutes per day or more of moderate to vigorous intensity aerobic activity with vigorous muscle-strengthening and bone-strengthening activities at least three days per week
- Most adults with diabetes to engage in 150 minutes or more of moderate to vigorous intensity physical activity per week, spread over at least three days per week with no more than two consecutive days without activity (or for younger and more physically fit adults, shorter-duration, high-intensity, or interval training may be sufficient)
- Adults with diabetes to engage in two to three sessions/week of resistance exercise on nonconsecutive days
- Older adults with diabetes to engage in flexibility training and balance training two to three times per week
- Sedentary persons with diabetes to increase activities such as walking, yoga, housework, gardening, swimming, and dancing above baseline
- All adults to decrease the amount of time spent in daily sedentary behavior and to interrupt periods of sitting every 30 minutes to improve blood glucose
(ElSayed et al., 2023f, 2023g)
To have a substantial role in treating diabetes, aerobic and resistance exercise must be regular and long-term. Therefore, exercise must fit realistically into the patient’s life. Duration and frequency of exercise are important in order to improve and maintain glycemic control along with weight management. Aerobic activity should last at least 10 minutes, with the goal of 30 minutes/day or more on most days of the week, with no more than two consecutive days without exercise (ElSayed et al., 2023f; Meneghini, 2020).
However, many individuals with type 2 diabetes struggle to meet the 150-minute recommendation for exercise per week. According to the CDC (2022a), only 23.8% of individuals with diabetes meet the recommended goal of 150 minutes of physical activity per week. In one study, text messages and phone calls resulted in an improvement in daily step count over a 12-month period. Such individualized approaches and encouragement are often required to help individuals with type 2 diabetes meet their exercise goals (ElSayed et al., 2023f).
Many patients with type 2 diabetes have lived sedentary lives before the time of their diagnosis. For this group of patients, an exercise schedule begins gradually, with short, regular walks or brief exercise sessions according to individual tolerance. Over time, the length and intensity of the exercise sessions are increased.
Depending on each patient’s individual functional status and exercise needs, they may need a more thorough evaluation by a physical therapist and/or occupational therapist prior to the start of an exercise program (ElSayed et al., 2023f).
CASE
Dwayne, a 55-year-old African American male with a family history of diabetes, is referred to the clinic for a diabetes workup. He reports having to urinate two or three times a night, frequent fatigue, weight gain of 7 pounds over the last month, and slight numbness in his feet. Upon examination, Dwayne is found to have a BMI of 31 kg/m2, a waist circumference of 108 cm (42.5 inches), an A1C level of 6.6%, and a fasting plasma glucose of 130 mg/dL. Further examination reveals evidence of hypertension (140/90 mmHg), dyslipidemia (HDL 22 mg/dL), and early signs of renal dysfunction, confirming the diagnosis of type 2 diabetes.
When presented with the results of his examination, Dwayne describes a sedentary lifestyle; frequent consumption of fried, fatty foods and soft drinks; infrequent consumption of fruits and vegetables; and frequent snacking.
The nurse counsels Dwayne on the importance of a healthy diet, proper nutrition, and regular exercise. The nurse, when consulting with the primary care provider, suggests that Dwayne would benefit from a referral to a dietitian for development of a diet plan as well as a physical therapist to formulate a therapeutic exercise program.
The patient is also scheduled to have a consultation with his primary care provider to discuss additional strategies and new medications that may be needed to manage his high blood pressure and dyslipidemia.
Medication Usage
One of the core ADCES self-care behaviors is medication usage. People with diabetes may take medication to lower their blood pressure and/or lower their blood glucose levels. The longer they have diabetes, the more they are likely to require medications to reduce their risk of complications. The ADCES recommends educating patients about the following self-care actions:
- Maintaining an updated list of medications they are currently taking and bringing it to all appointments
- Filling prescriptions in a timely manner in order to start taking them right away
- Establishing a routine in order to take the right medication at the right time
- Disposing of needles in a safe manner
(ADCES, 2023)
(See also “Medications” later in this course.)
PROBLEM-SOLVING
Problem-solving is an education topic recommended by the ADCES that enables patients to manage and cope with changes in the progression of their diabetes. The three steps of problem-solving include:
- Identify the problem
- Find solutions
- Take action
(ADCES, 2023)
Monitoring and Using Patient-Generated Health Data
Monitoring is a crucial part of effective self-care. In addition to monitoring their blood glucose, patients are taught how to self-monitor their blood pressure, weight, and sleeping patterns. Regular checkups are also an important part of the monitoring process to determine whether the individualized treatment plan is working or needs to be adjusted (ADCES, 2023).
HOME BLOOD GLUCOSE MONITORING
A key part of the patient education program is teaching patients how to check their blood glucose levels. Patients measure their blood glucose levels for two reasons:
- It provides a detailed record so that the healthcare team can recommend adjustments to meals, exercise, or medications.
- It gives the patient immediate feedback on how daily routines are affecting blood sugar levels.
Monitoring Frequency and Schedule
All patients with diabetes who are on insulin are taught to check their blood glucose levels at a variety of times. This builds a detailed record of the daily variation of glucose levels, which is especially useful while the initial treatment plan is being adjusted. Moreover, if patients monitor their blood glucose levels over an extended period of time, they will learn to recognize the feeling of hypoglycemia and help to distinguish it from other uncomfortable sensations.
The frequency with which a patient checks their glucose level varies and is an individualized determination. Patients who do not take insulin usually settle into a schedule of checking blood glucose levels once a day, either:
- First thing in the morning (fasting glucose)
- Before lunch, dinner, or snacks
- Before going to sleep
- When hypoglycemia is suspected and until they have normal blood sugar if they are hypoglycemic
- One to two hours after each meal
(ElSayed et al., 2023c)
Patients beginning insulin therapy are usually asked to monitor their blood glucose level four times a day until an optimal regimen of meals, exercise, and injections is established. After they have established a stable pattern, patients can reduce the number of blood tests as directed by their provider. Patients are advised to test their glucose level more frequently when their life pattern changes, when they experience symptoms of hypoglycemia, or when they develop another illness.
Using Glucose Meters
Blood glucose meters are pocket-sized, handheld electronic devices. Most home meters measure the glucose concentration in a drop of whole capillary blood from a finger prick. Some blood glucose meters also work with blood from other sites, such as the forearm or the palm area below the thumb. Home testing supplies come with a variety of features, and they are changing and improving continually. For example, some meters use lancing devices designed to be less painful, and others allow the user to add more blood to a testing strip that had a reading error (ElSayed et al., 2023i).
Using Continuous Glucose Monitoring Technology
Continuous glucose monitoring (CGM) monitors consist of a sensor that is attached to the arm and measures interstitial glucose. CGM allows real-time glucose monitoring to guide treatment decisions and is most often used for those patients who are on insulin therapy. Data from randomized controlled trials show that CGM technology has consistently demonstrated the ability to:
- Reduce A1C levels
- Prevent hypoglycemic episodes
- Reduce acute diabetes complications
- Prevent hospitalizations for hypoglycemia and hyperglycemia
(ElSayed et al., 2023i)
Patients using CGM technology are taught how to apply the sensor as well as how frequently to change it. Contact dermatitis is a potential side effect, so patch testing is conducted prior to the first use by patients with a known sensitivity to tape (ElSayed et al., 2023i).
Insurance coverage is not always available for CGM technology for all patients with type 2 diabetes. However, starting in 2023, the Centers for Medicare & Medicaid Services approved insurance coverage of CGM devices for all patients with diabetes who are on insulin therapy or who have had hypoglycemic events (AAFP, 2023). This change in policy makes CGM technology more accessible to the patients who can benefit from it the most.
Reducing Risk
Reducing risk is an ADCES-recommended self-care behavior and teaching topic for patients with diabetes. Quitting smoking, taking care of feet, and understanding signs and symptoms of hypoglycemia are examples of reducing risk to prevent complications (ADCES, 2023). (See also “Foot Problems” later in this course.)
HYPOGLYCEMIA RISK
It is important to educate all persons with type 2 diabetes, especially those who are on insulin therapy or sulfonylureas, about hypoglycemia (blood glucose <70 mg/dL) (Brutsaert, 2022). Severe hypoglycemia can cause unconsciousness and, if not corrected by the addition of glucose to the bloodstream, can eventually be fatal.
Hypoglycemia is less common for people with type 2 diabetes who are not receiving insulin therapy than for those who are receiving insulin therapy. But all diabetes patients should be taught to recognize the symptoms of hypoglycemia.
Clinicians must be aware of which individuals are at increased risk of experiencing hypoglycemia, including those:
- On insulin therapy
- With reduced renal or hepatic function
- Who have had diabetes for a long time
- Of older age and/or with frailty
- With cognitive impairment
- With hypoglycemia unawareness (i.e., are unaware or less sensitive to the signs and symptoms of hypoglycemia)
- With a history of severe hypoglycemic event
(ElSayed et al., 2023e)
Hypoglycemia Causes and Symptoms
For people on insulin therapy, missing a meal or exercising vigorously are the most common causes of hypoglycemia. Patients with type 2 diabetes who take antisympathetic drugs, such as beta blockers, are warned that these medications blunt the symptoms of hypoglycemia, making a potentially life-threatening situation less obvious.
All healthcare professionals who provide care to patients with diabetes must be able to quickly identify the signs and symptoms of hypoglycemia. One very common way to remember the symptoms of hypoglycemia is the phrase cold and clammy, need some candy. The mnemonic TIRED can also be a useful tool to remember the main signs and symptoms of hypoglycemia:
- Tachycardia (increased heart rate) and/or palpitations
- Irritability
- Restlessness/anxiety
- Excessive hunger
- Diaphoresis (sweating)
In addition to the main symptoms in the mnemonic TIRED, health professionals providing education to patients with type 2 diabetes teach their patients to recognize these additional symptoms of hypoglycemia:
- Chills and clamminess
- Weakness
- Shakiness
- Dizziness
- Faintness
- Nausea
- Anxiety
- Increased respiratory rate
- Headache
- Confusion
- Pale skin
- Blurred vision
- Seizures and coma
(Brutsaert, 2022; ADA, 2023d)
Treatment of Hypoglycemia
Prompt treatment of hypoglycemia is necessary to prevent serious complications such as brain damage, seizures, and coma. All individuals with type 2 diabetes are educated and given specific instructions about how to handle hypoglycemic episodes. Glucose is given immediately for a blood glucose level <70 mg/dL in order to prevent a further decreasing and a progression of symptoms. Carbohydrate sources high in protein (e.g., nuts) and complex carbohydrates are not recommended for the immediate treatment of hypoglycemia (Brutsaert, 2022; ElSayed et al., 2023f).
When symptoms of hypoglycemia are experienced, persons with diabetes are instructed to:
- Test blood glucose to determine if hypoglycemia is indeed the cause of symptoms.
- Treat blood glucose <70 mg/dL using “the rule of 15,” which is eating or drinking 15 grams of fast-acting carbohydrate (e.g., 1/2 cup fruit juice or sugared soda; four glucose tablets; or 1 tablespoon sugar, honey, or syrup), and then waiting 15 minutes before retesting.
- If the repeat test is still <100 mg/dL, treat with another 15 grams fast-acting carbohydrate and repeat the cycle.
All patients with type 2 diabetes are instructed to wear medical identification, such as a Medic-Alert bracelet. Some patients are also advised to have an emergency glucagon kit, and family and friends are taught how and when to give glucagon by inhalation or injection (ADA, 2023d).
CASE
Daniel is a bedside nurse working on a medical-surgical unit. His patient Henry is a 78-year-old male who is post-op day 1 for a hip replacement. Henry also has a secondary diagnosis of type 2 diabetes and has blood glucose checks ordered before meals and at bedtime.
A few hours after lunchtime, the visiting physical therapist calls Daniel to the room to check on Henry because something seems wrong. Daniel quickly goes to the patient room, where he finds Henry sitting up in bed looking very restless and sweaty. Henry is yelling irritably at the physical therapist: “What the heck is going on here? I’m starving!” Daniel applies the pneumonic TIRED and quickly assesses that Henry is tachycardic, irritable, restless, has excessive hunger, and is diaphoretic.
Daniel stays with the patient and calls his charge nurse to bring him the blood glucose testing supplies. Henry’s blood glucose result is 61 mg/dL. Daniel implements the hypoglycemic protocol standing orders and gives Henry a tube of 15-gram glucose gel. He also notifies the appropriate hospitalist regarding Henry’s condition. Fifteen minutes later, Henry’s blood glucose is 80 mg/dL. Daniel administers another tube of 15-gram glucose gel. Another 15 minutes later, Henry’s blood glucose is now 110 mg/dL, and the patient reports feeling much better. Daniel educates Henry on the signs and symptoms of hypoglycemia.
EXERCISE PRECAUTIONS
Physical activity is an integral part of the treatment plan for most patients with type 2 diabetes. Important considerations prior to implementing an exercise plan include education for individuals with type 2 diabetes who may need to monitor their blood glucose level during exercise and appropriate exercise precautions that must be followed for patients with certain complications of diabetes.
Depending on their medication regimen, persons with type 2 diabetes may need to monitor their blood glucose levels to assess for any fluctuations that occur with exercise. Physical activity may cause hypoglycemia during and for several hours after exercise. Factors that affect blood glucose levels during exercise include:
- Fitness level of individual
- Time of day
- Type of exercise/activity
- Prevailing glucose level prior to exercise
- Duration and intensity of exercise
The patient is instructed to monitor blood glucose levels and plan according to the following recommendations:
- Check blood glucose 15 to 30 minutes before exercise and every 30 minutes during a long workout.
- Check blood glucose again after exercise to see how exercise affects glucose levels. This is beneficial in planning for future exercise and whether medication adjustment or carbohydrate intake is needed.
- Always have a fast-acting carbohydrate food or drink available during exercise.
- If hypoglycemia occurs during exercise, stop exercising and eat one carbohydrate serving (15 grams). Check blood glucose in 15 minutes. Repeat as necessary to bring blood glucose to a safe range, then resume exercise.
- The effect of exercise on glucose levels can last for several hours after activity; be prepared for the possibility of delayed hypoglycemia.
- If rapid-, short-, or intermediate-acting insulin is being used as treatment, it may be necessary to decrease the dose of insulin taken prior to exercise by 10%–30% (long-acting insulin is not generally adjusted for exercise) as directed by the primary care provider.
- Whenever possible, exercise should be planned for times when insulin is not peaking.
(ElSayed et al., 2023f; Mayo Clinic, 2022)
Patients should not exercise:
- If blood glucose level is >250 mg/dL. It may be too dangerous to exercise safely, and a dose of insulin may be needed to reduce blood glucose level.
- If blood glucose is <100 mg/dL. Eat one or two carbohydrate servings (15 grams to 30 grams) and make sure the blood glucose level is between 100 and 250 mg/dL before starting exercise.
- When feeling ill.
- Prior to bedtime. This reduces the risk of hypoglycemia during the night. If evening exercise is necessary, patients may be instructed to take an extra carbohydrate serving before bed and wake up during the night to test blood glucose.
(ElSayed et al., 2023f; Mayo Clinic, 2022)
SMOKING CESSATION
Individuals with type 2 diabetes who smoke are at an increased risk of cardiovascular disease, premature death, and microvascular complications. They also have poorer glycemic outcomes compared to individuals who do not smoke. People who smoke should be counseled on the medical consequences of smoking and strongly encouraged to stop smoking. Since it is often difficult for smokers to quit on their own, they may find that formal programs that include support, counseling, and the availability of smoking cessation are helpful. E-cigarettes are also discouraged and are not considered an advisable method for smoking cessation (ElSayed et al., 2023f).
MEDICAL TREATMENT FOR TYPE 2 DIABETES
Although the treatment plan for a person with type 2 diabetes must be tailored to the individual, the typical progression begins with the lifestyle changes described above combined with metformin. Next, other oral or noninsulin injectable medications or insulin itself may be added, depending on how the person with type 2 diabetes is responding to the initial interventions. If the individual is not able to lose weight with lifestyle interventions, weight-loss medications and/or metabolic surgery may be considered (ElSayed et al., 2023c, 2023e).
Medications
The American Diabetes Association (ElSayed et al., 2023h) recommends that pharmacotherapy be started at the time of type 2 diabetes diagnosis as long as there are no contraindications. The treatment plan for most people with type 2 diabetes includes a mix of lifestyle interventions (weight loss, healthy nutrition, and exercise) along with oral or noninsulin injectable medications. Some people with type 2 diabetes may also require insulin, depending on how well their pancreas is functioning and whether or not they are able to reach their target A1C.
ORAL AND NONINSULIN INJECTABLE HYPOGLYCEMICS
Along with lifestyle changes, the addition of an oral hypoglycemic medication is often recommended as first-line therapy. There are many classes of oral and noninsulin hypoglycemic medications. Each one works in a different way to help the body lower blood glucose.
When choosing a single medication or combination of hypoglycemic drugs, it is useful to have records of fasting morning blood glucose levels. A patient-centered approach is then used to guide the choice of treatment, taking the following factors into consideration:
- Cardiovascular comorbidities
- Risk of hypoglycemia
- Impact on weight
- Cost
- Risk for side effects
- Patient preferences
(ElSayed et al., 2023h)
After 6 to 12 months of implementing and modifying an initial treatment plan, the frame of a long-term program takes shape. People with diabetes have a schedule of regular visits with their physician and other members of the diabetes care team. At each visit, the team reviews patient outcomes, which include A1C values, daily blood glucose records, and the development and/or progression of diabetic complications. The team also offers support with problems in daily healthcare routines. When lab values or the clinical picture suggest the treatment routine needs to be changed, the patient meets with the healthcare team more frequently until optimal health outcomes are again stabilized.
Metformin is usually recommended as a first choice because it is:
- Affordable
- Effective when combined with lifestyle changes
- Combinable with most other hypoglycemics if needed
- Rarely the cause of hypoglycemia
(ElSayed et al., 2023h)
Metformin counteracts insulin resistance by reducing the amount of glucose released by the liver and, to a lesser extent, by improving the ability of muscle cells to extract glucose from the circulation. Technically, metformin is antihyperglycemic, not hypoglycemic. It does not cause insulin to be released from the pancreas, and therefore it rarely causes hypoglycemia, even in large doses (Meneghini, 2020). Metformin commonly causes bloating, abdominal discomfort, and diarrhea, but these side effects often resolve with gradual dose titration (ElSayed et al., 2023h).
The initiation of metformin is given a three- to six-month trial. If this succeeds in reducing A1C values to <7%, the regimen is continued. The patient continues to be evaluated and A1C levels measured every three to six months. Treatment with metformin continues for as long as tolerated. If the three- to six-month trial does not lead to an A1C value <7%, the patient continues lifestyle changes along with additional medications (ElSayed et al., 2023h; Meneghini, 2020).
Additional classifications of oral and noninsulin injectable hypoglycemics are listed in the table below.
| Drug Class | Action | Side effects | Examples |
|---|---|---|---|
| (ElSayed et al., 2023h; Collins & Costello, 2023; Meneghini, 2020) | |||
| Glucagon-like peptide-1 (GLP-1) receptor agonists |
|
|
|
| Sodium-glucose cotransporter 2 (SGLT2) inhibitors |
|
|
|
| DPP-4 inhibitors |
|
|
|
| Thiazolidinediones |
|
|
|
| Sulfonylureas |
|
|
|
INSULIN THERAPY
Eventually, many adults with type 2 diabetes will require insulin therapy. When the combination of lifestyle changes and hypoglycemic agents cannot reduce A1C values below 7%, insulin therapy may be initiated. Insulin therapy often begins as an adjunct to oral therapy, such as metformin, plus a daily injection of long-acting basal insulin (ElSayed et al., 2023h; Meneghini, 2020).
Typically, type 2 diabetes is diagnosed when a person has already lost about half of their normal insulin-producing ability. Over the years, the ability of pancreatic beta cells to secrete insulin continues to decrease in persons with type 2 diabetes. When the pancreas can only secrete 20% to 30% of the normal amount of insulin, a patient begins to need insulin therapy. The majority of people with type 2 diabetes begin to need insulin less than 10 years after their diagnosis. This process should be explained in an objective manner to patients. It is important not to threaten patients with insulin therapy or use insulin therapy as an indication that the patient failed or is being punished (ElSayed et al., 2023h; Meneghini, 2020).
The initial aim of insulin therapy is to increase the basal supply of insulin (i.e., that which is essential for maintaining fundamental vital activities), and this is usually done with intermediate- or long-acting insulin. Some patients may also require prandial (meal-associated) therapy with short- or rapid-acting insulin (ElSayed et al., 2023h; Meneghini, 2020).
If A1C targets are not achieved with insulin therapy, treatment can be intensified via addition of an agent from a different drug class, such as a GLP-1 receptor agonist. The overall objective is to achieve and maintain control of blood glucose and to change interventions when therapeutic goals are not being met (ElSayed et al., 2023h).
The idea of taking insulin injections can cause anxiety in patients, but by reducing the levels and durations of their hyperglycemic episodes, patients can delay or prevent the otherwise debilitating complications of the disease. Insulin can be administered in more convenient delivery forms such as injection pens, pumps, or inhaled rapid-acting insulin (if not contraindicated, since inhaled insulin could result in a decrease in lung function). When insulin is successfully incorporated into the treatment of poorly controlled diabetes, glycemic control is more achievable (Meneghini, 2020; ElSayed et al., 2023h).
Types of Insulin
Three characteristics distinguish the available forms of insulin: how fast they act, when they peak, and how long they act.
- Regular insulin is short acting. It begins acting in 30 to 60 minutes, reaches its peak of action in 2 to 3 hours, and acts for 5 to 8 hours. An example of regular insulin is human regular.
- Insulin analogues are rapid acting. They begin acting in 5 to 15 minutes, reach their peak of action in 1 hour, and act for 3 to 6 hours. Examples of rapid-acting insulins are lispro, aspart, and inhaled insulin.
- Intermediate-acting insulin begins acting in 2 to 4 hours, reaches a peak of action in 6 to 10 hours, and acts for 10 to 16 hours. An example of intermediate-acting insulin is human NPH.
- The effects of long-acting insulins can last for up to a day. Examples of long-acting insulins are glargine (Lantus) and detemir (Levemir).
(Meneghini, 2020; ElSayed et al., 2023h)
Most often, people with type 2 diabetes start with one long-acting insulin injection. As time goes on, they may need to switch to a more complex insulin regimen to match the daily changes in blood glucose levels (i.e., high after meals and low during the night). However, individuals with type 2 diabetes may need simplified insulin regimens as they get older due to a reduction in their ability to self-manage (ElSayed et al., 2023h).
HERBS, SUPPLEMENTS, AND DRUG COMPLICATIONS
There are claims that multiple herbs and spices have blood glucose–lowering properties that make them helpful in the treatment of people with type 2 diabetes. For most supplements, however, there is insufficient evidence to support a beneficial effect on diabetes or its complications. Such supplements also have the potential to interact with other medications, including antidiabetic agents, thus increasing the risk of severe hypoglycemia and other complications. Herbs and other supplements should never be added to the diet without the knowledge and approval of the patient’s primary healthcare provider.
Plant-based therapies that have been shown, in some studies, to have antidiabetic properties include:
- Garlic
- Bitter melon
- Vitamin C
- Vitamin D
- Vitamin E
- Ginger
(Yedjou et al., 2023)
CASE
Barb, a 60-year-old female patient whose type 2 diabetes had been well controlled with sulfonylurea therapy, presents with a fasting plasma glucose level of 68 mg/dL. After prompting from the nurse, Barb reveals that she has been supplementing her diet lately with aloe vera. The nurse counsels Barb that, although they may produce positive effects, natural supplements may lower blood sugar to dangerous levels when used in combination with prescribed antidiabetic medications. Therefore, the use of natural remedies must be closely monitored. The nurse recommends discontinuing the aloe vera until Barb’s glucose levels return to normal. The nurse also advises Barb to consult again with the clinician and/or a dietitian before resuming supplementation.
APPROVED WEIGHT-LOSS MEDICATIONS
The U.S. Food and Drug Administration has approved five medications that can be used more than 12 weeks in patients with a BMI ≥27 kg/m2 and type 2 diabetes. These medications improve glycemic control and are intended to be used in conjunction with lifestyle modifications. The medications approved by the FDA for the treatment of obesity in adults are shown in the table below.
| Name | Type | Dosing | Side Effects |
|---|---|---|---|
| (ADA, 2022b) | |||
| Phentermine/topiramate ER | Sympathomimetic amine anorectic/antiepileptic combination | 7.5 mg/46 mg each day |
|
| Orlistat | Lipase inhibitor | 60 mg three times daily (OTC) or 120 mg three times daily (Rx) |
|
| Naltrexone/bupropion ER | Opioid antagonist/antidepressant combination | 16 mg/180 mg twice daily |
|
| Liraglutide | Glucagon-like peptide 1 receptor agonist | 3 mg each day |
|
| Semaglutide | Glucagon-like peptide 1 receptor agonist | 2.4 mg once weekly |
|
The effectiveness, side effects, and safety of the selected weight-loss medication are evaluated on a monthly basis for the first three months and then every three months after that. Effective therapy results in a >5% weight loss in the first three months. If this result is not achieved, the treatment is stopped and other treatment options are considered (ADA, 2022b).
Metabolic Surgery
When nonsurgical methods such as lifestyle modifications and medications do not produce durable weight loss, metabolic surgery may be considered as a treatment option.
Metabolic surgery is a term referring to surgical procedures for obesity treatment. It is also called bariatric surgery, weight-loss surgery, or metabolic/bariatric surgery. Metabolic surgery may be considered if the patient has tried monitored dieting, exercise regimens, and medications without success. A significant body of evidence shows that metabolic surgery achieves superior glycemic control and reduction of cardiovascular risk factors in patients with type 2 diabetes who are obese compared with various lifestyle/medical interventions (ADA, 2022b).
The American Diabetes Association recommends surgery as an option to treat individuals with type 2 diabetes and a BMI ≥40 kg/m2 (BMI >37.5 kg/m2 in Asian Americans), especially if their diabetes or associated comorbidities are difficult to control with lifestyle changes and drug therapy. Surgery may also be considered as an option for adults with type 2 diabetes and BMI between 30.0 and 34.9 kg/m2 (27.5–32.4 kg/m2 in Asian Americans) who do not achieve sustained weight loss and improvement in comorbidities through other methods (ADA, 2022b).
BARIATRIC SURGERY ACCREDITATION
Metabolic surgery is recommended to be performed in high-volume medical centers that use a multidisciplinary team (physician, psychologist, physical therapist, occupational therapist, and dietitian) trained in diabetes, obesity, and bariatric surgery. Patients making the decision to have surgery should be made aware of quality and standards for centers that perform bariatric surgery (ADA, 2022b).
There is one national accreditation standard for bariatric surgery centers: the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). This accreditation is important because it provides an objective and measurable way for a center to demonstrate that it offers high-quality care to patients in the setting of a multidisciplinary team approach (MBSAQIP, 2023).
TYPES OF BARIATRIC SURGERY
Bariatric surgery assists with weight loss in two ways: restriction of the amount of space in the stomach (limiting intake of food) and malabsorption by shortening or bypassing the small intestine (reducing absorption). Examples of common bariatric surgery procedures are listed below. The majority of bariatric surgery procedures in the United States are the vertical sleeve gastrectomy and Roux-en-Y gastric bypass procedures (ADA, 2022b).
Vertical sleeve gastrectomy (gastric sleeve) is a procedure involving the surgical removal of approximately 80% of the stomach. The remaining portion of the stomach is formed into a smaller tubelike structure. This smaller stomach restricts the amount of food intake and decreases the production of ghrelin (a hormone that regulates the appetite).

Gastric sleeve. (Source: National Institutes of Health.)
Roux-en-Y gastric bypass (gastric bypass) is one of the most common bariatric surgical procedures, in which the surgeon creates a small pouch at the top of the stomach and attaches a narrow portion of the small intestine directly to the pouch, limiting the amount of food a person can eat as well as the amount of calories and nutrients absorbed.

Gastric bypass (Roux-en-Y). (Source: National Institutes of Health.)
Laparoscopic adjustable gastric banding (lap banding) is a procedure that involves placing a band with an inflatable balloon around the upper part of the stomach. The band restricts the size of the stomach as well as narrows the opening to the rest of the stomach. A port placed under the skin in the abdominal area is connected and used to inflate or deflate the band to adjust the size. This procedure restricts the amount of food intake, with an early feeling of fullness (ADA, 2022b; Merck Manual, 2022).
Gastric banding. (Source: National Institutes of Health.)
POSTSURGICAL CARE
Hospital care of the post-op patient with metabolic surgery is complex, and nurses must be knowledgeable about the procedures and risks to continually assess their patients. Major complications occur in 2%–6% of patients who have metabolic surgery. Patients who undergo these procedures are at risk for blood clots, blocked intestines, and leaking from surgical connections. Post-op orders must be strictly followed, and might include allowing the patient only 30 mL of ice chips at a time.
Patients are educated that it could take up to four weeks before they may eat any solid foods. They will be on a limited liquid diet for the first two weeks and will need to gradually build up the amount they are allowed to intake at a time. Unfortunately, some patients eat more than recommended after metabolic surgery, which can be dangerous. Long-term risks of metabolic surgery include vitamin and mineral deficiencies, dumping syndrome (when food moves from the stomach to the small intestine too quickly), and severe hypoglycemia (ADA, 2022b; Merck Manual, 2022).
After surgery, lifelong lifestyle support and medical monitoring is important to improve outcomes. Early mobilization is an important part of long-term recovery, with assistance from occupational therapists to teach activities of daily living and physical therapists to create and monitor a regular exercise and strengthening program (ADA, 2022b).
HYPERGLYCEMIA-RELATED ILLNESSES AND COMPLICATIONS
People with diabetes face both acute and chronic health threats. Acute complications include diabetic ketoacidosis and hyperglycemic hyperosmolar state. (See also “Hypoglycemia” earlier in this course.) Diabetes also continually injures tissues microscopically, and as these microscopic injuries accumulate, they lead to observable chronic problems such as heart disease or kidney failure.
Acute Complications
Before the discovery of insulin, most people with diabetes died of a condition known as diabetic coma, which came on suddenly and was fatal by the second or third day. Typically, this fatal condition was characterized by dehydration and precipitated by the occurrence of some other disease. Today, diabetic coma is called diabetic ketoacidosis.
Diabetic ketoacidosis and hyperosmolar hyperglycemic state (also called hyperosmotic hyperglycemic nonketotic state) are two emergency conditions that threaten people with type 1 and type 2 diabetes. Both conditions involve a high level of blood glucose that leads to dehydration beyond the body’s ability to cope. The person becomes tired and weak, is thirsty and urinates excessively, and often has an altered mental state, ranging from confusion to coma. Dehydration causes hypotension and acute renal failure, and if not treated with IV fluids and insulin, the condition leads to serious electrolyte abnormalities, brain injury, and death (Brutsaert, 2022).
DIABETIC KETOACIDOSIS
Diabetic ketoacidosis develops when there is so little insulin that the body begins to use fat as a major fuel. Diabetic ketoacidosis is seen primarily in people with type 1 diabetes, although some people with type 2 diabetes may develop the condition.
Diabetic ketoacidosis is characterized by acidic ketones (the result of fat metabolism) in the blood and urine, and ketones can be smelled on the patient’s breath, giving a fruity or acetone-like odor. The resulting acidosis—a drop in blood pH below 7.3 (normal pH is 7.38 to 7.44)—causes the body to adopt a deep, sighing pattern of respiration called Kussmaul respirations. Diabetic ketoacidosis also produces nausea, vomiting, and abdominal pain (Brutsaert, 2022).
HYPEROSMOLAR HYPERGLYCEMIC STATE
Hyperosmolar hyperglycemic state (HHS) develops when there is sufficient insulin for the body to use glucose as a fuel but there is not enough insulin to keep blood glucose levels in a safe range. HHS is primarily seen in older adults with type 2 diabetes.
Unlike diabetic ketoacidosis, HHS produces no ketones. The condition is caused directly by very high blood glucose levels, typically >600 mg/dL. Without acidic ketones, there is no Kussmaul breathing and usually no abdominal distress. HHS tends to develop slowly, over days or weeks, and by the time it is apparent, the patient may already be confused or stuporous.
Both diabetic ketoacidosis and HHS are emergencies and are treated in the same way. The patient is given insulin to lower the hyperglycemia and fluids to reverse the dehydration. The blood electrolyte levels are corrected, and for diabetic ketoacidosis, the pH balance of the body is shifted back toward normal. Typically, both conditions are precipitated by another recent stressor, so this problem, too, must be identified and corrected. The patient is usually monitored in an intensive care unit (Brutsaert, 2022).
DIABETES COMORBIDITIES
Diabetes comorbidities are medical conditions that people with diabetes are more likely to develop than people of the same age who do not have diabetes. Diabetic comorbidities differ from diabetes-related complications and include:
- Cancer (specifically cancers of the liver, pancreas, endometrium, colon/rectum, breast, and bladder)
- Cognitive impairment and dementia
- Nonalcoholic fatty liver disease
- Obstructive sleep apnea
- Periodontal disease
(ElSayed et al., 2023e)
Chronic Complications
Because continual hyperglycemia is the cause of the chronic complications of diabetes, any reduction of average blood glucose levels (i.e., A1C values) reduces the chances of developing chronic complications. Prolonged hyperglycemia has a number of deleterious effects.
MICROSCOPIC INJURY
Two types of microscopic cell and tissue damage seem to be involved in most of the long-term complications of diabetes.
When there is excess glucose in the bloodstream, glucose molecules stick indiscriminately to proteins in a process called glycosylation. (For example, excess glucose binds to hemoglobin, and this is the basis of the A1C index of hyperglycemia.) Higher blood glucose levels produce more glycosylated proteins, and these glycosylated proteins tend to cross-link (bind together) into abnormal complexes. The complexes then add to atherosclerotic plaque, damage kidneys, and disrupt the structure of extracellular matrices.
Excess glucose also amplifies the amount of certain rarely produced chemicals in the body. These chemicals include sorbitol, diacylglycerol, and fructose-6-phosphate, all of which, in sufficient quantities, are detrimental to the normal functioning of cells.
Both types of molecular problems damage blood capillaries, endothelial cells of larger blood vessels, and nerves. The accumulation of these microscopic injuries leads to the macroscopic damage that produce the long-term complications of diabetes (Mota et al., 2020).
MACROSCOPIC INJURY
Over the years, the continual hyperglycemia of diabetes takes its toll on tissues everywhere in the body. The chronic complications of diabetes are caused by the macroscopic damage that occurs after the accumulation of 10 to 20 years of microscopic damage. In type 2 diabetes, hyperglycemia may have been present for many years before the disease is recognized. Therefore, many people with type 2 diabetes may already have macroscopic damage when they are first diagnosed (ADA, 2021; Mota et al., 2020).
In the United States, the major long-term health problems from diabetes are:
- Heart disease
- Stroke
- Hypertension
- Blindness
- Kidney disease
- Nervous system disease
- Amputations
- Dental disease
- Complications of pregnancy
- Death
(ADA, 2021; CDC, 2022a)
The most common long-term complications of diabetes are damage to arteries, kidneys, eyes, nerves, and feet.
CARDIOVASCULAR DISEASES
Persons with diabetes are at increased risk for coronary artery disease (CAD) and cardiovascular disease (CVD), including an increase in the incidence of myocardial infarction (MI) and stroke. Medical management of cardiovascular risk factors is a key component for reducing risk in patients with type 2 diabetes. This includes assessment, management, and ongoing monitoring of hypertension, hyperlipidemia, and obesity.
Diagnostic testing for CAD and CVD is considered in people with any atypical cardiac signs or symptoms or an abnormal ECG. Some individuals may require additional screening with stress tests or echocardiogram (ElSayed et al., 2023d).
People with type 2 diabetes develop atherosclerotic coronary artery disease more frequently than people without diabetes because elevated blood glucose levels can damage small cardiovascular blood vessels. Atherosclerosis causes myocardial infarction, heart failure, stroke, and insufficient circulation to the feet. Today, over 60% of the people with type 2 diabetes die from some form of cardiovascular disease (Mota, 2020).
Coronary heart disease and stroke are the two predominant types of cardiovascular disease. When people with diabetes take steps to control their blood pressure, cholesterol, and other cardiovascular risk factors, they can reduce their risk of CVD or possibly slow its progression (Meneghini, 2020).
Patients with type 2 diabetes are screened annually for signs, symptoms, and risk factors of cardiovascular disease. Recommendations suggest a referral to cardiology for evaluation and cardiac stress tests for patients with diabetes who also have:
- Cardiac symptoms
- An abnormal resting ECG
- Peripheral or carotid artery disease
- Autonomic neuropathy affecting the cardiovascular system
(Meneghini, 2020)
By controlling their blood glucose levels, people with type 2 diabetes reduce the likelihood of having heart and artery problems. The risk of cardiovascular disease can be reduced still further by reducing high blood pressure. In addition, aspirin therapy (75–162 mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased risk for cardiovascular problems and as a secondary prevention strategy in those with diabetes and a history of arteriosclerotic CVD (ElSayed et al., 2023d).
Hypertension
Individuals with diabetes are advised to keep their blood pressure below 130/80 mmHg. Lower blood pressure goals are considered for patients based on individual benefits and risks. Even with lifestyle changes, most people with type 2 diabetes and hypertension require antihypertensive medications (often two or more drugs) to reach this target. Patients with hypertension and type 2 diabetes also perform home monitoring of their blood pressure. Maintaining a low blood pressure may help prevent other complications of diabetes such as vision loss and kidney failure (Meneghini, 2020; ElSayed et al., 2023d).
Lowering of blood pressure with regimens based on a variety of antihypertensive agents, including ACE inhibitors, angiotensin receptor blockers (ARBs), diuretics, and calcium channel blockers, has been shown to be effective in reducing cardiovascular events (ElSayed et al., 2023d).
Dyslipidemia
Dyslipidemia increases the risk of developing cardiovascular diseases. Fasting lipid levels of people with type 2 diabetes are screened yearly and unhealthy lipid levels treated.
In terms of reducing cardiovascular risk, the primary goal is a fasting triglyceride level of <150 mg/dL and fasting HDL cholesterol levels of >40 mg/dL in men and >50 mg/dL in women. People who already have some form of cardiovascular disease should aim for a lower LDL cholesterol level of <70 mg/dL (ElSayed et al., 2023d).
Statin drugs are the most effective medications for controlling total and LDL cholesterol. The treatment plan for patients with diabetes who are over the age of 40 includes a statin medication and also considers daily aspirin therapy, which can prevent the aggregation or clumping of platelets in the blood from forming clots that can block blood flow to the heart or the brain (Meneghini, 2020).
Prothrombotic State
In the prothrombotic state (a condition in which the blood clots inside blood vessels more easily than normal), unnecessarily high levels of clotting molecules in the bloodstream increase a person’s risk for developing coronary artery disease and stroke. A low dose (75–162 mg/day) of enteric-coated aspirin may be recommended for some people with type 2 diabetes to help prevent cardiovascular disease. Patients with aspirin allergies, bleeding disorders, recent gastrointestinal bleeding, or liver disease should not take aspirin (ElSayed et al., 2023d; Meneghini, 2020).
DIABETIC NEPHROPATHY
Diabetic nephropathy is a common complication of diabetes and the leading cause of chronic kidney disease in the United States. Diabetic nephropathy can progress to end-stage renal disease, and many people with end-stage renal disease have type 2 diabetes.
Diabetes injures those cell membranes in the kidney that are responsible for filtration and absorption of fluids and molecules. One of the earliest indicators of membrane damage is seen in the kidney glomeruli, the first of the filtration sites, which become slightly leaky and allow small amounts of protein into the urine. There is a small amount (30–300 mg/24 hours) of protein that abnormally appears in the urine (called moderately increased albuminuria).
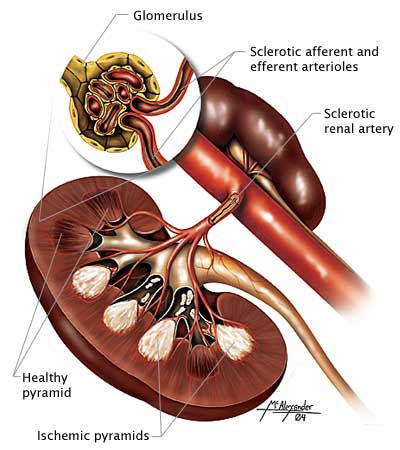
A cross-section of the kidney showing ischemic pyramids and sclerotic arteries and arterioles. (Source: Illustration by Jason McAlexander, © Wild Iris Medical Education, Inc.)
Without treatment, the ability of the glomeruli to keep protein out of the urine declines, and eventually the person has severely increased albuminuria (the excretion of a significant amount [>300 mg/24 hours] of protein). While glomeruli are losing their ability to exclude large molecules such as proteins from the urine, they are also becoming less able to filter fluid, and the glomerular filtration rate (GFR) declines. At the same time, blood pressure begins to rise (Levy & Nessen, 2022a).
Treatment for Diabetic Nephropathy
The American Diabetes Association (ElSayed et al., 2022d) recommends that patients with type 2 diabetes check two indicators of kidney functioning annually:
- Blood should be tested for serum creatinine levels, which is used to estimate the glomerular filtration rate.
- Urine should be tested to assess albumin excretion.
In addition, blood pressure is measured at each checkup.
By controlling all three major risk factors (blood glucose levels, blood pressure, and blood lipid levels), people with type 2 diabetes can delay the development of kidney problems. People with diabetes who already have microalbuminuria can slow its progression to diabetic nephropathy by the same interventions. When kidney problems have progressed to albuminuria and a declining GFR, it is best to consult a kidney specialist.
By itself, good control of serum glucose levels will not prevent kidney problems in people with type 2 diabetes. On the other hand, when part of a regimen that targets all the major risk factors, glycemic control does slow the onset and progression of diabetic nephropathy.
Controlling blood pressure is also an effective way to delay or prevent kidney problems in people with type 2 diabetes since hypertension accelerates the development of kidney damage.
DIABETIC RETINOPATHY
Diabetic retinopathy is the primary reason for new cases of blindness in adults ages 20–74 years. Individuals with type 2 diabetes are at risk for the development of retinopathies, cataracts, and glaucoma. It takes approximately five years for retinal damage to develop after the onset of hyperglycemia. Serious eye damage results from long-term diabetic injury to capillaries and small blood vessels of the retina (ADA, 2023d).
The retina viewed through a scope showing damage due to diabetes, including light exudate splotches, mini-hemorrhages, and areas of neovascularization. (Source: Illustration by Jason McAlexander. © Wild Iris Medical Education, Inc.)
Hyperglycemia causes damage to the retina over time. Tiny blood vessels leading to the retina are damaged and blocked by hyperglycemia. The body compensates by creating new blood vessels to grow along the retina, along with fibrous connective tissue. This is considered proliferative diabetic retinopathy, which can later cause blindness (NEI, 2022)
Regular dilated-eye exams are important to screen and monitor for any eye complications. For effective treatment, detecting retinal damage early is critical. People with diabetes undergo a full (dilated) eye examination by an ophthalmologist or a trained optometrist when they are first diagnosed and every year thereafter (unless a less frequent schedule is recommended by an ophthalmologist). A patient with kidney damage may already have retinopathy, so kidney damage is a warning that the patient should be seen by an ophthalmologist (ADA, 2023d).
DIABETIC NEUROPATHY
Diabetic neuropathies may be present even in those newly diagnosed with diabetes since elevated glucose levels may have been present for a number of years before a formal diabetes diagnosis. Nerve damage is a common complication for people with diabetes.
Peripheral sensory and motor neuropathies injure the longest nerves first and show up in the feet before the hands. Over the years, peripheral neuropathies slowly move proximally. Sensory problems include paresthesias, numbness, and pain; motor problems include reduced deep tendon reflexes and muscle weakness (ADA, 2023d).
Nerve damage can decrease a patient’s ability to sense actual pain, and at the same time, it can cause phantom burning pain, especially at night. Sometimes, motor nerves are affected and muscles or reflexes are weakened. When autonomic nerves are damaged, the patient can have symptoms ranging from impotence to digestive problems to dizziness on standing.
Diabetic neuropathies can take many forms. The two most common are generalized nerve injuries (called diabetic distal symmetrical polyneuropathy) and diabetic autonomic neuropathy.
Distal Symmetrical Polyneuropathy (DSPN)
Symptoms of distal symmetrical polyneuropathy show up at the ends of the longest nerves first. Typically, DSPN begins with unusual sensations such as tingling and numbness in the toes and feet. Over time, the paresthesias and numbness slowly move upward until they are distributed like socks on the feet, ankles, and legs. Before the problem reaches the knees, long nerves elsewhere in the body become affected, beginning at the fingers.
Eventually, the hands and lower arms have sensory reductions in a distribution like a pair of gloves. This “stocking-glove” pattern of sensory deficits is followed by decreased reflexes in the feet and ankles and by weakness in the muscles that spread the toes (Meneghini, 2020).
Screening for DSPN is done by testing the ability of the patient to sense the vibration of a 128-Hz tuning fork in the toes of both feet and also the ability to sense the pressure of a standardized 10-g diabetes monofilament on the bottoms of the toes. The Achilles tendon reflexes at the ankles are checked as well as each foot for skin lesions, soft tissue injuries, and joint damage (ADA, 2023d).
Autonomic Neuropathy
When diabetes damages the autonomic nerves, the symptoms vary and can affect any of the internal organs. Possible symptoms include:
- Cardiovascular: resting tachycardia (≥100 bpm), orthostatic hypotension (a drop in systolic blood pressure of 20 mmHg or more upon standing), fainting, exercise intolerance
- Gastrointestinal: difficulty swallowing, slow emptying of the stomach, highly erratic or labile blood glucose levels, weak response to hypoglycemia, constipation, alternating bouts of diarrhea and constipation, nocturnal diarrhea, fecal incontinence
- Genitourinary: impotence, reduced vaginal lubrication, inability to empty the bladder, recurrent urinary tract infections
- Skin: reduced sweating of hands and feet (which may make the patient’s skin dry and itchy)
(ElSayed et al., 2023e; ADA, 2023d; Meneghini, 2020)
All individuals with type 2 diabetes are monitored for autonomic neuropathy. To screen for autonomic neuropathy, patients are assessed for the cardiovascular, gastrointestinal, genitourinary, or skin symptoms listed above. Patients may also experience orthostatic hypotension; the resting blood pressure of patients is taken and compared with blood pressure after standing (ADA, 2021).
By improving glycemic control, neurologic symptoms can sometimes be reduced, but there are no cures for the nerve damage of diabetes; treatments for diabetic neuropathy only alleviate symptoms. Symptoms of diabetic neuropathy are treated individually:
- Poor sensation in the feet is managed by educating the patient about foot care, by limiting impact exercises, and by regular foot exams every 3–6 months. The patient may benefit from a referral to a podiatrist or advanced practice nurse specializing in managing diabetic foot care. Additional management includes referral to a physical therapist for evaluation of the extent of sensation loss, functional limitations caused by diminished balance and/or proprioception, or the need for special footwear and/or assistive devices (such as a cane or walker) when needed. An occupational therapist consultation with the patient and family is also important to address issues inherent to the home setting and activities of daily living (Meneghini, 2020).
- Pain from neuropathy is treated by a specialist, who may recommend a medication or another regimen appropriate to the particular patient.
- Orthostatic hypotension is evaluated by a neurologist. Therapy usually includes having the patient sleep with the head of the bed elevated, avoid sudden posture changes by sitting or standing slowly, and wear full-length elastic stockings.
- Diarrhea from autonomic neuropathy is evaluated by a GI specialist. Sometimes, the diarrhea resolves on its own, but if not, it may respond to antidiarrheal medications or to antibiotic therapy. In other cases, diarrhea can be caused by impaired neural control of sphincters and result in fecal incontinence.
- Constipation is usually treated with a stimulant laxative, such as senna.
- Gastroparesis (decreased stomach motility, which delays digestion of food), bladder dysfunction, and impotence is usually improved by medications.
CASE
Alessandro is a 53-year-old male patient being treated for newly diagnosed type 2 diabetes, hypertension, and dyslipidemia. He has been referred for an initial evaluation with a physical therapist due to several recent falls that occurred at the factory where he is employed.
In the initial patient interview, Alessandro states that he has fallen on the job at least five times during the past six months. Two falls occurred when he tripped over a large wooden crate and three occurred when he may have tripped on something but “wasn’t sure what.” In addition to describing the falls, Alessandro also reports feelings of slight numbness, tingling, and decreased sensation in his feet over the past several months. He states that he has not exercised regularly for over 10 years but that he previously enjoyed playing basketball and golf.
Alessandro tells the therapist that he understands the importance of overall fitness in helping him to best manage his diabetes and that he would like to be able to be more active in the sports he used to enjoy. He states that he has been hesitant to participate in sports, however, due to fear of falling. Alessandro describes his goals for physical therapy as being able to perform his job without fear of falling and to find meaningful and enjoyable fitness activities for himself.
The physical therapist completes an initial evaluation of his functional status, which reveals the following pertinent objective information about Alessandro:
- Range of motion and manual muscle testing within functional limits for upper and lower extremities
- Berg Balance score of 37/56, indicating significant compromise of static and dynamic balance
- Ability to stand on his right foot for 7 seconds without losing balance and on his left foot for 3 seconds before losing balance
- Ability to maintain Romberg position for approximately 25 seconds before losing balance
- Inability to maintain sharpened Romberg position without external support
- Decreased proprioception at both ankles and diminished two-point discrimination on the plantar surfaces of both feet
- Frequent loss of balance when attempting to walk on uneven or soft surfaces
Together, the physical therapist and Alessandro develop the following goals to address both his current functional deficits and his long-term personal objectives:
Short-Term Goals
- Alessandro will be independent and compliant with a home exercise program that addresses static and dynamic balance, skin inspections to both feet, and proprioceptive activities.
- Alessandro will improve his Berg Balance score by at least 3 points.
Long-Term Goals
- Alessandro will demonstrate a Berg Balance score of 49 or above to ensure that he is safe with independent ambulation and at minimal risk of falling.
- Alessandro will demonstrate consistently safe ambulation over all surfaces (including soft, uneven, or sloped surfaces) without loss of balance.
- Alessandro will successfully navigate his work environment without loss of balance or falls for a period of 30 days.
- Alessandro will be independent and active in a long-term plan of preferred fitness activities for which he has been medically cleared for participation (such as swimming, water polo, walking, or cycling) in order to optimize his overall activity and fitness level as a component to helping him most effectively manage his medical condition.
The physical therapist recommends a plan of care to include outpatient physical therapy two times weekly for a period of 4–6 weeks in order to address static and dynamic balance training, proprioceptive activities, training in foot inspections, workplace and community safety awareness training, and structuring and tailoring of an overall, long-term fitness plan.
FOOT PROBLEMS
It has been estimated that 20% of hospital admissions of patients with diabetes are for foot problems. Over the years, damage to capillaries and small blood vessels reduces the ability of the microscopic circulation to deliver oxygen to the feet of people with diabetes. In persons with diabetes, the feet and ankles can suffer from reduced microvascular and macrovascular circulation, poor healing, and peripheral neuropathy (damage to the nerves outside of the brain and spinal cord). In addition, many people with diabetes develop atherosclerotic peripheral vascular disease, which impedes the overall circulation to their feet (Meneghini, 2020; ADA, 2021).
People with diabetic neuropathy can have muscle weakness and a poor sense of position. Diabetic neuropathy is also associated with diminished ankle range of motion, which can directly affect gait and balance. For these reasons, people with diabetic neuropathy tend to injure their feet, ankles, and legs. Any damage to their sensory nerves will make these frequent small injuries less likely to be noticed. To compound the problem, diabetic ischemia of the lower legs slows the healing of injuries and encourages infections.
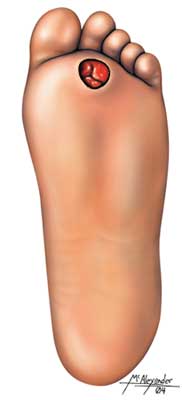
Decreased sensation from peripheral neuropathy can lead to ulcers. Daily inspections are important to address small abrasions and sores before they develop into ulcers. (Source: Illustration by Jason McAlexander. © Wild Iris Medical Education, Inc.)
At each visit, the patient’s nails, skin, and joints of both of the ankles and feet are examined (Meneghini, 2020). The skin on the feet and toes is assessed for erosions, ulcers, and infections. Additionally, assessment includes checking for capillary refill under the nails of the toes and whether the feet are cool and pale. The ankle and foot joints are assessed for deformities and injuries (ElSayed et al., 2023e).
Testing includes assessing the foot and ankle pulses for the ability to feel pressure and vibration and two-point discrimination to test sensation in the toes. The clinician assesses the patient as they walk, looking for uneven gait and checking shoes for uneven wear and for locations of excess pressure. The patient is asked whether they have other problems walking, such as intermittent claudication, weakness, or imbalance. Another important consideration for self-care is to determine whether the patient can safely trim toenails. A referral to a podiatrist for regular foot care and nail trimming can be very beneficial to preventing problems (Meneghini, 2020).
Patients with diabetes are warned about the extra risks that foot injuries pose for them. A physical therapist or other clinician working with a patient who has existing neuropathy or poor circulation instructs the patient and family on the proper way to inspect the feet for any injuries (e.g., with the use of a handheld mirror) on a regular basis (ADA, 2021). Patients are encouraged to examine their feet every day and counseled on how to care for their skin and toenails and to choose appropriate footwear. If patients have difficulty examining and caring for their feet, someone else (a family member, nurse, podiatrist, or physical therapist) is enlisted to help. Foot care is part of the initial education of all patients diagnosed with diabetes (Meneghini, 2020).
WHAT PATIENTS NEED TO KNOW ABOUT FOOT CARE
- Cut toenails straight across and inspect the feet daily for cuts, scratches, blisters, and corns.
- Clean the feet daily with warm water and mild soap followed by thorough drying.
- Use a gentle moisturizer cream regularly (e.g., Eucerin or lanolin).
- Avoid prolonged soaking, strong chemicals (e.g., Epsom salts or iodine), and any home surgery.
- Avoid hazards such as going barefoot, extreme heat or cold, and wearing tight socks or shoes.
- Wear well-fitted walking shoes that distribute pressure evenly on the foot and provide proper cushioning.
(ADA, 2021; Meneghini, 2020)
The most common cause of foot injury is excess pressure on the skin over bony prominences of the feet and ankles (such as metatarsal heads, calcaneus, and malleoli). To ease pressure points, well-fitted athletic shoes are preferable to dress shoes or shoes with hard soles. For feet with insufficient circulation or poor sensation, special shoes with individualized internal molds may be needed in order to evenly distribute pressure during walking. Diabetic footwear includes a high, wide toe box (to maximize space and reduce pressure), removable insoles to insert orthotics if needed, and rocker soles and heel counters to provide support and stability (Physiopedia, 2023). These patients should see a podiatrist along with a physical therapist to address strategies and management to decrease injury to the feet (Meneghini, 2020).
Wounds on feet with neuropathy or poor circulation cannot be treated casually. Even small wounds must be thoroughly examined, cleaned, debrided, and then reexamined daily. Patients may be unaware of diabetic pressure injuries and other wounds, as they may have lost sensation. Antibiotics are used at the first signs of infection. Walking and other foot pressures are minimized while the wounds are healing. Soft-tissue infections are treated aggressively with hospitalization and IV antimicrobial therapy.
When a diabetic foot becomes pale, pulseless, and painful, it is an emergency, and a surgeon is consulted.
AMPUTATION
At times, if a patient’s condition is not well managed, the chronic limb and foot problems may result in damaged and deformed joints and nonhealing skin ulcers. When soft tissues or bones become infected, the destruction may become severe enough to require amputation of toe(s) and/or the entire limb. The highest risk of amputation is in people who have had diabetes for more than 10 years and in whom microscopic tissue damage has already resulted in eye or kidney problems (Meneghini, 2020).
The following conditions are associated with an increased risk of amputation:
- Peripheral neuropathy
- Altered biomechanics (limited mobility, bony deformities, nail infections)
- Peripheral arterial disease
- History of nonhealing ulcers or amputations
(Cifu, 2020; Meneghini, 2020)
If a patient requires amputation, initial surgery and recovery are managed in an inpatient setting with a team approach. Rehabilitation with support from physical and occupational therapists and prosthetists during the pre- and postoperative period are vital to the patient.
Preoperative
Preoperative education includes a discussion of:
- Process and importance of rehabilitation after surgery
- Process of phantom pain
- Healing process
- Prosthetic casting, fitting, and training
(Cifu, 2020)
Postoperative
Postoperative rehabilitation goals include:
- Pain management
- Incisional healing
- Postoperative limb positioning and wrapping (to prevent contractures and help manage edema)
- Physical therapy:
- Bed mobility
- Transfers
- Lower extremity range of motion and strengthening
- Mobility training with appropriate assistive device (walker, wheelchair, cane, etc.)
- Balance (seated, standing, walking, stairs)
- Progressively increasing activities to tolerance
- Discharge planning (assistive device recommendations/training, home exercise program, determining need for further therapies at home, etc.)
- Occupational therapy:
- Assisting with ADLs
- Assessing need for adaptive equipment in the home
- Evaluating home safety and recommendations for modifications if needed
- Developing energy conservation strategies
- Upper extremity stretching and strengthening
(Cifu, 2020)
The goal of rehabilitation therapy after amputation is to have the patient successfully transfer back to their baseline or home environment. After surgical recovery, the patient may need to remain inpatient within a more intensive and supervised rehabilitation environment, or may be able to participate in either home-based or outpatient rehabilitation. Regardless of the rehabilitation environment upon inpatient discharge, prompt follow-up with a physical therapist, an occupational therapist, and a prosthetist is important in order to continue to meet patient needs (Cifu, 2020).
The patient is assessed for the appropriate prosthetic, including a preparatory limb, after a period of wound healing has taken place. Transition to a prosthetic includes assessing arm strength, balance, bed-to-chair transfers, and contralateral limb strength. Prosthetic training and exercises focus on skin care, sock management, gait training, range of motion, stretching and strengthening, and managing care at home with the prosthesis (activities of daily living such as dressing, bathing, and household chores) as well as a continued program of home exercises (Cifu, 2020).
CASE
Shanelle is a 64-year-old female with a history of type 2 diabetes and obesity who presents to the clinic for a checkup. On examination of the patient’s feet, the physician discovers a diabetic ulcer 4 cm in diameter on the sole of her right foot. The physician asks Shanelle if she has any pain in her foot, and Shanelle states that her feet feel just fine. The physician explains the diagnosis and orders wound care to be done at the clinic three times per week while the diabetic ulcer heals.
The nurse conducts follow-up education with Shanelle, including counseling on proper foot care, the need to use a mirror to check her feet daily, and wearing well-fitted shoes that provide proper cushioning. The nurse explains that diabetic foot ulcers can take months to heal, and it is important that Shanelle come to her wound care appointments to avoid further complications, including amputation.
Exercise Precautions and Diabetic Complications
The care management team screens each person with type 2 diabetes for health problems that must be accommodated prior to the start of an exercise program. Very few problems prohibit adding more physical activity to the daily life of a person with diabetes, but certain problems put special constraints on those activities.
Cardiovascular disease. Before a sedentary patient with diabetes and cardiovascular risks starts a new exercise program, the patient undergoes a medical exam, including a stress test to assess cardiac function. This may not be needed for young, otherwise healthy people with diabetes. If the test shows cardiovascular problems, it is still possible to create a gradually increasing exercise plan, with the patient warned not to overexert and to watch for symptoms of angina, including chest, jaw, or arm tightness or pain, and palpitations or shortness of breath (ElSayed et al., 2023f).
Untreated retinopathy. Proliferative diabetic retinopathy (damage to the retina) or severe nonproliferative retinopathy puts a person at risk for vitreous hemorrhages or retinal detachment. Vigorous exercise should be avoided by individuals with these conditions, and they should be examined by an ophthalmologist before adding intense exercise into the management plan (ElSayed et al., 2023f).
Peripheral neuropathy. A patient who lacks the ability to fully sense injury to the feet, ankles, and legs can damage skin and joints without realizing it. Peripheral neuropathy can also often affect balance and equilibrium. Therefore, patients with diabetes who have peripheral neuropathy may benefit from working closely with a physical therapist or exercise physiologist to incorporate an individualized plan of care for rehabilitation that is appropriate for their specific situation. Patients with significant peripheral neuropathy should not participate in strenuous exercise such as prolonged walking, treadmill use, jogging, or step exercise unless specifically instructed and monitored by an appropriate healthcare professional (ElSayed et al., 2023f).
Autonomic neuropathy. Damage to the autonomic nervous system can cause reduced or inappropriate responses to exercise. Patients with diabetes who have autonomic neuropathy are given a thorough cardiac examination before beginning a new exercise program (ElSayed et al., 2023f).
General Rehabilitation Management and Type 2 Diabetes
Patients with type 2 diabetes who have chronic, progressive complications or are recovering from procedures or medical/surgical interventions often require structured rehabilitation for a successful recovery. The most common chronic effects that patients with type 2 diabetes may experience include peripheral neuropathy, orthostatic hypotension, vision changes, and cardiac decompensation (Cifu, 2020).
The key to a successful rehabilitation program is individualization. Rehabilitation specialists, including physical therapists and occupational therapists, start by evaluating the patient’s baseline physical condition and any disabilities or impairments that are present due to either deconditioning or chronic effects of diabetes. The patient’s interests and motivation for improving their condition is an important component as well. Initial goal setting involves the patient and family to work toward mutual goals for progress.
Patients with type 2 diabetes may also require assistive devices to improve functional activities of daily living. They may have reduced sensation, pain, weakness, gait disturbances, balance problems, and/or pain. Mechanical mobility aids (such as wheelchairs, walkers, or canes) may help reduce pain and lessen the impact of physical disability. Properly fitted hand or foot/ankle braces may help compensate for muscle weakness or alleviate nerve compression. Orthopedic shoes may improve gait disturbances and help prevent foot injuries in people with a loss of pain/sensation. Patients with neuropathy have been shown to benefit from aquatic therapy focusing on gait training, strength, and balance control.
For patients with peripheral neuropathy, weight-bearing exercise should be modified to reduce the load on the extremities. Examples of activities that can be done include water aerobics, riding a stationary bike, and using an elliptical machine. Non-weight-bearing exercise is recommended for patients with diabetes who have open sores on their feet or a foot injury.
Patients with orthostatic hypotension are advised to be proactive when changing from a supine to standing position by moving slowly and holding onto a chair or bed for 30 seconds after standing to minimize their risk for falling. Patients may also be advised to wear supportive compression stockings to increase venous return. Consultation with a cardiologist is recommended before starting a vigorous exercise program with patients whose blood pressure drops over 30 mmHg from supine to standing (Harris-Hayes et al., 2020).
If a patient has an existing cardiac condition, gentle daily exercise is often encouraged to increase and maintain the ability to perform ADLs. These may include chair exercises, arm exercises, and other nonstrenuous movements such as tai chi or chair yoga.
For a patient who would like to work on increasing balance and strength, resistance exercises (such as leg extensions, chest press, and rowing), functional exercises (such as stand-sit-stand and stair climbing), and balance training (heel-toe walking, standing on one leg, etc.) may be incorporated with appropriate guidance from therapy professionals (Cifu, 2020).
CONCLUSION
Type 2 diabetes continues to be a major health condition that challenges the global population. Lifestyle changes and poor nutrition habits have contributed to the increasing prevalence of type 2 diabetes. Additional awareness of the risk factors and physiology of diabetes are important factors for healthcare providers to understand. Patients at risk should have regular screening and early implementation of recommended lifestyle modifications to prevent the onset and progression of type 2 diabetes.
As healthcare providers working with patients who are diagnosed with type 2 diabetes, it is important to understand the common complications that may occur as well as the most effective educational strategies to support the health and wellness of patients with type 2 diabetes and their families.
RESOURCES
American Diabetes Association
800-DIABETES (800-342-2383)
Association of Diabetes Care & Education Specialists (ADCES)
Diabetes (CDC)
Diabetes Care Publication (ADA)
Standards of Care in Diabetes—2023
eAG/A1C conversion calculator (ADA)
Glucose-lowering medications in type 2 diabetes (ADA)
National Institute of Diabetes and Digestive and Kidney Diseases
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
American Academy of Family Physicians (AAFP). (2023). Medicare expands coverage of continuous glucose monitoring. https://www.aafp.org
American Diabetes Association (ADA). (2021). Microvascular complications and foot care: Standards of medical care in diabetes—2021. Diabetes Care, 44(Suppl. 1), S151–67. https://diabetesjournals.org
American Diabetes Association (ADA). (2022a). Fast facts: Data and statistics about diabetes. https://professional.diabetes.org
American Diabetes Association (ADA). (2022b). Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of medical care in diabetes—2022. Diabetes Care, 45(Supp. 1), S113–24. https://diabetesjournals.org
American Diabetes Association (ADA). (2022c). Chronic kidney disease and risk management: Standards of care in diabetes—2022. The Science of Diabetes Self-Management and Care, 45(1), S175–84. https://diabetesjournals.org
American Diabetes Association (ADA). (2023a). eAG/A1C conversion calculator. https://professional.diabetes.org
American Diabetes Association (ADA). (2023b). Recipes & nutrition: Eat good to feel good. https://diabetes.org
American Diabetes Association (ADA). (2023c). Get smart on carbs: Carb counting and diabetes. https://diabetes.org
American Diabetes Association (ADA). (2023d). Blood glucose testing and management: Hypoglycemia (low blood glucose). https://diabetes.org
American Physical Therapy Association (APTA). (2020). Physical therapy guide to diabetes. https://www.choosept.com
Association of Diabetes Care & Education Specialists (ADCES). (2023). Using the ADCES7 self-care behaviors to improve your health. https://www.diabeteseducator.org
Brutsaert EF. (2022). Diabetes mellitus (DM). Merck Manuals. https://www.merckmanuals.com
Centers for Disease Control and Prevention (CDC). (2022a). National diabetes statistics report: Estimates of diabetes and its burden in the United States. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022b). Calculating BMI using the English system. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022c). Diabetic ketoacidosis. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2023). What is diabetes? https://www.cdc.gov
Cifu DX. (2020). Braddom’s physical medicine and rehabilitation (6th ed.). Elsevier.
Cleveland Clinic. (2022). Subcutaneous fat. https://my.clevelandclinic.org
Collins L & Costello RA. (2023). Glucagon-like peptide-1 receptor agonists. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Davis J, Fischl AH, Beck J, Browning L, Carter A, Condon J, Dennison M. et al. (2022). 2022 national standards for diabetes self-management education and support. The Science of Diabetes Self-Management and Care, 48(1), 44–59. https://diabetesjournals.org
Diabetes.co.uk. (2022). Insulin resistance. http://www.diabetes.co.uk/insulin-resistance.html
Diabetes.co.uk. (2023a). Drug induced diabetes. https://www.diabetes.co.uk/drug-induced-diabetes.html
Diabetes.co.uk. (2023b). Secondary diabetes. https://www.diabetes.co.uk/secondary-diabetes.html
Diabetes Education Online. (2023). Insulin basics. https://dtc.ucsf.edu
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023a). Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S19–40. https://diabetesjournals.org
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023b). Improving care and promoting health in populations: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S10–18. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023c). Prevention or delay of type 2 diabetes and associated comorbidities: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S41–8. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023d). Cardiovascular disease and risk management: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S158–90. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023e). Comprehensive medical evaluation and assessment of comorbidities: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S49–67. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023f). Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2023. Diabetes Care, 46(Suppl. 1), S68–96. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023g). Children and adolescents: Standards of care in diabetes—2023. Diabetes Care, 46(Suppl. 1), S230–53. https://www.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023h). Pharmacologic approaches to glycemic treatment: Standards of care in diabetes—2023. Diabetes Care, 46(Suppl. 1), S140–57. https://pubmed.ncbi.nlm.nih.gov
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. (2023i). Diabetes technology: Standards of care in diabetes—2023. Diabetes Care, 46(Supp. 1), S111–27. https://diabetesjournals.org
Ghosh S, Mahalanobish S, & Sil PC. (2022). Diabetes: Discovery of insulin, genetic, epigenetic and viral infection mediated regulation. Nucleus, 65(2), 283–97.
Harris-Hayes M, Schootman M, Schootman JC, & Hastings MK. (2020). The role of physical therapists in fighting the type 2 diabetes epidemic. The Journal of Orthopaedic and Sports Physical Therapy, 50(1), 5–16. https://www.jospt.org/doi/pdf/10.2519/jospt.2020.9154
Levy SM & Nessen M. (2022a). Obesity. Merck Manuals. https://www.merckmanuals.com
Levy SM & Nessen M. (2022b). Metabolic syndrome (Syndrome X; insulin resistance syndrome). Merck Manuals. https://www.merckmanuals.com
Mayo Clinic. (2022). Diabetes and exercise: When to monitor your blood sugar. https://www.mayoclinic.org
MedlinePlus. (2023). Drug-induced low blood sugar. https://medlineplus.gov
Meneghini L. (2020). Medical management of type 2 diabetes (8th ed.). American Diabetes Association.
Merck Manual. (2022). Weight-loss surgery (bariatric surgery). https://www.merckmanuals.com
Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). (2023). Bariatric surgery. https://www.facs.org
Mota RI, Morgan SE, Bahnson EM. (2020) Diabetic vasculopathy: Macro and microvascular injury. Current Pathobiology Reports, 8(1), 1–14.
Nakrani MN, Wineland RH, & Anjum F. (2023). Physiology, glucose metabolism. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
National Eye Institute (NEI). (2022). Diabetic retinopathy. https://www.nei.nih.gov
National Heart, Lung, and Blood Institute (NHLBI). (n.d.). Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. https://www.nhlbi.nih.gov
Pastore I, Assi E, Ben Nasr M, Bolla AM, Maestroni A, Usuelli V, Loretelli C, et al. (2021). Hematopoietic stem cells in type 1 diabetes. Frontiers in Immunology, 12, 694118. https://www.ncbi.nlm.nih.gov
Physiopedia. (2023). Diabetes mellitus type 2. https://www.physio-pedia.com
Ridwanto M, Indarto D, & Hanim D. (2020). Factors affecting fasting blood glucose in patients with type 2 diabetes mellitus. International Journal of Nutritional Sciences, 5(1), 13–8. https://ijns.sums.ac.ir/article_46362_0cbc84b20331a50a6dc29bb7c76f63d4.pdf
Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, Isaacs SD, et al. (2023). American Association of Clinical Endocrinology consensus statement: Comprehensive type 2 diabetes management algorithm—2023 update. Endocrine Practice, 29, 305–40.
U.S. Food and Drug Administration (U.S. FDA). (2022). How to understand and use the nutrition facts label. http://www.fda.gov
Wondmkun YT. (2020). Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes, Metabolic Syndrome and Obesity, 13, 3611–6.
World Health Organization (WHO). (2023). Diabetes. http://www.who.int/mediacentre/factsheets/fs312/en/
Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, Latinwo L, & Tchounwou PB. (2023). The management of diabetes mellitus using medicinal plants and vitamins. International Journal of Molecular Sciences, 24(10). https://www.ncbi.nlm.nih.gov
Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, Hong D, Tian S, & Sun C. (2020). Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutrition & Diabetes, 10(1), 38. https://pubmed.ncbi.nlm.nih.gov





Customer Rating
4.9 / 850 ratings