Dementia: Alzheimer's Disease Patient Care
Online Continuing Education Course

Course Description
Alzheimer's disease and dementia continuing education course. Covers pharmacologic and medical therapies, the role of rehabilitation in caring for patients with Alzheimer's, strategies for addressing the effects of AD, and ways to support families and caregivers. Earn 10 contact hours with this Alzheimer's CEU. Newly updated bestseller course!
Course Price: $49.00
Contact Hours: 10
Case Management Contact Hours: 7.25
Pharmacotherapeutic Hours: 0.5
Course updated on
January 14, 2025
"Great Course! I am a Geriatric Nurse Specialist and thought I knew all there was to know about Alzheimers, but I learned so much more with this course!" - Laura Bryant, RN
"Very comprehensve with sound practical application based on evidence-based practice." - Lisa, RN in California
"Excellent course. I also work closely with clients with Alzheimer's disease and their families, and I recently lost an uncle to Alzheimer's. Very Helpful!" - Tina, RN in West Virginia
"Great course! Liked the brain images provided. Comprehensive!" - Siobhan, OT in North Carolina
Dementia: Alzheimer’s Disease Patient Care
Copyright © 2025 Wild Iris Medical Education, Inc. All Rights Reserved.
LEARNING OUTCOME AND OBJECTIVES: Upon completion of this continuing education course, you will have increased your knowledge of evidence-based guidelines for delivering appropriate therapeutic interventions to persons with Alzheimer’s disease, their family members, and caregivers. Specific learning objectives to address potential knowledge gaps include:
- Summarize the epidemiologic and societal impacts of Alzheimer’s disease.
- List risk factors and possible preventive measures for Alzheimer’s disease.
- Identify the signs, symptoms, and diagnostic steps for the disease.
- Discuss available pharmacologic and medical therapies.
- Summarize strategies in the rehabilitation and care of persons with Alzheimer’s disease.
- Identify interventions in managing problem behaviors.
- Describe effective support for families and caregivers.
- Discuss ethical, legal, and end-of-life considerations.
TABLE OF CONTENTS
- Introduction
- Scope of the Disease
- What Is Alzheimer’s Disease?
- Alzheimer’s Disease Signs and Symptoms
- Diagnosing Alzheimer’s Disease
- Pharmacologic and Medical Management
- Rehabilitation for Persons with Dementia
- Supportive Care for the Person with Alzheimer’s Disease
- Learning to Manage Problem Behaviors
- Caring for the Caregivers
- Ethical and End-of-Life Considerations
- Conclusion
- Resources
- References
INTRODUCTION
Alzheimer’s disease (AD) is named after Dr. Alois Alzheimer, who in 1906 noticed changes in the brain tissue of a woman who had died of an unusual mental illness. Her symptoms included memory loss, language problems, and unpredictable behavior. Following her death, he examined her brain and found many abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary, or tau, tangles).
These plaques and tau tangles in the brain are some of the main physical features of AD. Another feature is the loss of connections between neurons that transmit messages between different parts of the brain and from the brain to muscles and organs of the body (NIA, 2023a).
Alzheimer’s disease is one of a group of disorders called dementias, which are brain failures characterized by progressive cognitive and behavioral changes. The most common forms of dementia are:
- Alzheimer’s disease
- Vascular dementia
- Multi-infarct dementia
- Subcortical vascular dementia
- Stroke-related dementia
- Frontotemporal dementia (Pick’s disease)
- Mixed dementia (a combination of two or more types)
Other rarer conditions that can result in dementia include:
- Atypical Alzheimer’s disease
- Cadasil (a rare inherited form of vascular disease)
- Corticobasal syndrome (CBS)
- Creutzfeldt-Jakob disease (CJD)
- HIV-associated neurocognitive disorder (HAND)
- Huntington’s disease
- Normal pressure hydrocephalus (NPH)
- Progressive supranuclear palsy (PSP)
(Alzheimer’s Society, 2024a)
Alzheimer’s disease results from a complex pattern of abnormal changes, develops slowly, and gradually worsens. The course of Alzheimer’s and the rate of decline vary from person to person. Alzheimer’s disease can be present for many years before there are clinical signs and symptoms of the disease. On average, a person with Alzheimer’s lives for four to eight years after diagnosis. However, some may live for as many as 20 years.
Alzheimer’s disease is reported as the sixth leading cause of death in the United States. However, studies have found that it is underreported as an underlying cause of death. It is the only cause among the top 10 that cannot be prevented or cured. However, currently some treatments can help manage symptoms and slow disease progression for a period of time (Alzheimer’s Association, 2024a).
Historical Perspective
“Senile dementia”—the loss of memory and other intellectual faculties that occurs in older adults—was recognized in the time of Hippocrates. In the centuries that followed, this condition was thought to be simply a result of old age, commonly called hardening of the arteries. Diseases of old age, however, were considered unimportant until the second half of the 19th century. Prior to this period, people in the United States lived an average of 50 years and few reached the age of greatest risk for Alzheimer’s disease. For this reason, the disease was considered rare, and there was little scientific interest in it.
This changed as the average lifespan increased and Alzheimer’s became more common in people aged 70 and older. During this period of time, advancements in medicine and the ability to look inside the brain gave the medical community the realization that diseases could be the cause of this deterioration.
| (Alzheimer’s Association, 2024b) | |
| 1906 | German psychiatrist Alois Alzheimer first described the pathology of the disease after using staining techniques to identify amyloid plaques and neurofibrillary tangles in the brain associated with the symptoms of senile dementia. |
| 1910 | The disease was labeled Alzheimer’s disease by Emil Kraepelin. |
| 1931 | After the invention of the electron microscope, it became possible to conduct further study of the brain by viewing actual brain cells, opening the door to research into many areas of brain disorders, including Alzheimer’s disease. |
| 1968 | The Lawton Instrumental Activities of Daily Living Scale was developed to measure cognitive function at baseline and to identify improvement or deterioration over time. |
| 1976 | Alzheimer’s disease was recognized as the most common form of dementia. |
| 1980 | The Alzheimer’s Association was founded. |
| 1983 | National Alzheimer’s Disease Month was declared. |
| 1984 | Beta-amyloid was identified as forming Alzheimer’s disease’s characteristic plaques, which cause reduced neurologic function. A nationwide infrastructure for Alzheimer’s research was established by the National Institute on Aging. |
| 1986 | Tau protein was identified as forming Alzheimer’s disease’s characteristic neurofibrillary tangles. |
| 1987 | The first Alzheimer’s drug trial (tacrine) was begun. The first deterministic Alzheimer’s gene, amyloid precursor protein (APP), was discovered. |
| 1993 | The first Alzheimer’s disease risk factor gene was identified, called APOE4. The first Alzheimer’s drug, tacrine (Cognex), was approved by the U.S. Food and Drug Administration (FDA). |
| 1994 | President Reagan announced he had been diagnosed with Alzheimer’s disease. The first World Alzheimer’s Day was held. |
| 1996 | FDA approved donepezil (Aricept), a cholinesterase inhibitor, for treating Alzheimer’s-type dementia. |
| 1999 | Report published showing that injecting transgenic “Alzheimer’s” mice with beta-amyloid prevents the animals from developing plaques and other Alzheimer’s-like brain changes. |
| 2000 | FDA approved rivastigmine (Exelon), a cholinesterase inhibitor, for treating all stages of Alzheimer’s disease. |
| 2001 | FDA approved galantamine (Razadyne), a cholinesterase inhibitor, for treating mild to moderate Alzheimer’s disease. |
| 2003 | FDA approved memantine, an N-methyl-D-aspartate (NMDA) antagonist that reduces certain types of brain activity by binding to NMDA receptors and blocking the activity of glutamate, which in Alzheimer’s disease can overstimulate nerve cells and kill them. |
| 2004 | A new imaging agent known as Pittsburgh Compound B (PiB) was produced to be used with positron emission tomography for early detection of Alzheimer’s. Alzheimer’s Disease Neuroimaging Initiative was begun to share research data worldwide. |
| 2009 | An effort was begun to standardized biomarkers for Alzheimer’s disease. |
| 2011 | Alzheimer’s disease advanced to become the sixth leading cause of death in the United States and the fifth leading cause of death for persons over the age of 65. Canadian scientists used a technique known as deep brain stimulation (applying electricity to regions of the brain) to reverse Alzheimer’s disease-related memory loss. Annual assessment for cognitive impairment for all Medicare recipients was implemented as part of an annual wellness visit. President Obama signed the National Alzheimer’s Project Act into law, a framework for a national strategic plan. |
| 2012 | Scientists at University College London discovered that specific antibodies that block the function of a related protein (Dkk1) are able to completely suppress the toxic effect of beta-amyloid on synapses. The first major clinical trial for prevention of Alzheimer’s disease was begun. |
| 2013 | International Genomics of Alzheimer’s Project researchers identified new genetic risk factors for Alzheimer’s disease. |
| 2014 | FDA approved donepezil combined with memantine (Namzaric) for treatment of moderate to severe Alzheimer’s disease. Rates of death caused by Alzheimer’s disease were found to be much higher than reported on death certificates. |
| 2015 | A UCLA study identified three distinct subtypes of Alzheimer’s disease: inflammatory, noninflammatory, and cortical (associated with significant zinc deficiency). Research began to determine if they have different underlying causes and respond differentially to potential treatments. |
| 2017 | An historic $400 million increase for federal Alzheimer’s disease research funding was signed into law, bringing annual funding to $1.4 billion. |
| 2018 | Dementia Care Practice Recommendations were developed to help professional care providers deliver optimal quality, person-centered care. |
| 2021 | Aducanumab (Aduhelm), the first therapy to address the underlying biology of Alzheimer’s disease, received accelerated approval by the FDA for limited use. |
| 2023 | Lecanemab (Leqembi), which addresses the underlying biology of AD, was approved for treatment of early AD. Donanemab (Kisunla) was approved; it removes beta-amyloid from the brain. |
| 2024 | Aducanumab (Aduhelm) was discontinued by its manufacturer, Biogen. |
Scientists continue the search for answers regarding causes, diagnoses, and treatments for Alzheimer’s disease, but developing new treatments for Alzheimer’s disease has proven difficult. Some challenges in developing new treatments include:
- Most drugs fail during testing.
- Brains are almost impenetrable and are protected by the blood-brain barrier.
- Treating a symptom isn’t treating a disease.
- There is inadequate funding for Alzheimer’s research.
- Scientists aren’t sure what causes Alzheimer’s disease.
(Brookshire, 2024)
SCOPE OF THE DISEASE
Alzheimer’s Disease Worldwide
Every three seconds someone in the world develops dementia, and every year there are nearly 10 million new cases. Worldwide, more than 55 million people are living with Alzheimer’s and other dementias, over 60% of whom are in low- and middle-income countries. That number is expected to increase in 2030 to 78 million and in 2050 to 139 million. Dementia is one of the major causes of disability and dependency among older people globally. Dementia is currently the seventh leading cause of death, and 65% of dementia-related deaths are in women (WHO, 2024).
A systematic review and meta-analysis done in 2020 showed that the prevalence of dementia was higher in Europe and North America than in South America, Asia, and Africa. China has surpassed all other countries to become the nation with the highest number of dementia patients. Currently more than 15 million people ages 60 and above in China have dementia, accounting for a quarter of all dementia patients worldwide. Of this number, 9.83 million have Alzheimer’s disease. The disease is now affecting people in China at a younger age, with 21.4% being below the age of 60. AD and other dementias have become an increasingly serious public and social problem (Lv et al., 2023; Global Times, 2023).
A recent study reveals that two small Indigenous groups in the Bolivian Amazon have among the lowest rates of dementia in the world, at around 1% in people ages 60 and older (Miller, 2022).
Alzheimer’s Disease in the United States
It is estimated that as many as 6.9 million Americans age 65 and older have Alzheimer’s disease. As the size of the U.S. population ages 65 and older continues to grow, so too will the number and proportion of Americans with AD and other dementias. By 2050, the number of people age 65 and older with Alzheimer’s may reach a projected 12.7 million unless there is a medical breakthrough to prevent or cure the disease (Alzheimer’s Association, 2024a).
The states with the highest prevalence of Alzheimer’s disease are in the east and southeast regions, with the highest in Maryland (12.9%), New York (12.7%), and Mississippi (12.5%). States with the highest number of people with AD were California, Florida, and Texas. Among larger counties, those with the highest prevalence of AD were Miami-Dade County in Florida, Baltimore City in Maryland, and Bronx County in New York (Alzheimer’s Association, 2024c).
BY AGE
Following is the distribution of Alzheimer’s by age in the United States:
- 65–74 years: 26.4%
- 75–84 years: 38.6%
- 85+ years: 35.4%
(Statista, 2024a)
BY SEX
Almost two thirds of Americans with AD are women. Of the 6.9 million people ages 65 and older with AD, 4.2 million are women (11%) and 2.7 million (9%) are men. The main reason for this is that women live longer than men and older age is the biggest risk factor for this disease. Studies have been unclear whether those of female sex are more likely to develop dementia than those of male sex (Alzheimer’s Association, 2024a). (See also “Sex” under “Etiology and Risk Factors of Alzheimer’s Disease” later in this course.)
BY RACE/ETHNICITY
African Americans are about two times more likely than White people to have Alzheimer’s and other dementias but only 34% more likely to have a diagnosis. They are also more likely to be diagnosed in later stages. Hispanics are about one and one half times more likely than White people to have Alzheimer’s and other dementias but only 18% more likely to have a diagnosis (Alzheimer’s Association, 2024d).
As many as 1 in 3 Native American older adults will develop Alzheimer’s or some other form of dementia. Between 2020 and 2060, the number of American Indian/Alaska Native individuals age 65 and older living with dementia is projected to increase fourfold. More than one third of Native Americans say they do not expect to live long enough to develop Alzheimer’s, and more than half (53%) believe that significant memory or cognitive losses are a normal part of aging (Alzheimer’s Association, 2024e).
BY EDUCATION LEVEL
Research has found a high educational level to be associated with a 30% lower risk of Alzheimer’s compared with a low educational level. Combining genetic risk and education categories, individuals with a low genetic risk and a high educational level had a more than 90% lower risk of AD compared to those with a high genetic risk and low educational level (Li et al., 2023).
MORBIDITY AND MORTALITY
Before a person with Alzheimer’s dies, they live through years of morbidity as the disease progresses.
Between 2019 and 2020, the total number of deaths from Alzheimer’s disease increased 10.5%, with COVID-19 being a significant contributor. In 2022, AD was the seventh-leading cause of death in the United States, with more than 120,000 deaths and an age-adjusted mortality rate of 28.9 per 100,000 people. This was a nearly 7% decline from 2021, when deaths from AD had more than doubled between 2000 and that year. Among Americans ages 65 and older, AD is the fifth-leading cause of death (Alzheimer’s Association, 2023a).
Alzheimer’s disease is associated with excess comorbidity, including hypertension, diabetes (types 1 and 2), cardiovascular disease, and depression. There is evidence that risk factors common to comorbidities and AD, such as chronic inflammation, can place individuals with comorbidities at increased risk of developing AD. The interplay between comorbidities and development and progression of AD, however, remains incompletely understood (Lanctôt et al., 2023).
UNDERDIAGNOSIS OF ALZHEIMER’S
A 2019 study indicated that Alzheimer disease may be an underlying cause of five to six times as many deaths as currently reported. This is due to the fact that prevalence studies are designed so everyone in the study undergoes evaluation for dementia. Outside of research settings, however, a substantial portion of those who would meet the diagnostic criteria for Alzheimer’s and other dementias are not diagnosed by a physician. As result, a large portion of those with dementia may not know they have it.
Almost all existing Alzheimer’s prevalence studies are based on identification of clinical symptoms and do not rely on the brain changes believed to be responsible for the disease. Both autopsy studies and clinical trials have found that 15%–30% of those who meet the criteria based on symptoms did not have Alzheimer’s-related brain changes. Therefore, estimates could be up to 30% lower than estimates based only on symptoms (Alzheimer’s Association, 2024a).
Financial Impact of Alzheimer’s
Medical and long-term care for Alzheimer’s disease and related dementias impose a large economic burden on both individuals and societies. Nationally, in 2022 the estimated healthcare costs associated with Alzheimer’s disease treatment were $321 billion, with costs projected to exceed $1 trillion by 2050. Medicare and Medicaid cover approximately two thirds of these costs. The remaining costs, including out-of-pocket expenditures, are typically borne by the patients and their families, private insurance, health-maintenance or managed-care organizations, and uncompensated care.
The total lifetime cost of care for a patient with dementia was estimated at $412,936 in 2022 dollars, with 70% of those costs borne by family caregivers in the form of unpaid caregiving and out-of-pocket expenses for items ranging from home health support to medications. An estimated 11.3 million family and unpaid caregivers of individuals with AD or other dementias provided approximately 16 billion hours of informal (unpaid) assistance valued at approximately $271.6 billion.
The average total annual costs for Medicare beneficiaries ages 65 and older with AD or other dementias have been estimated to be $41,757, which is about three times higher than for those without dementia. The average Medicaid costs for Medicare beneficiaries with AD have been found to be 22 times higher than for those without AD ($64,478 vs $291).
In 2021, nursing home care averaged $95,000 to $108,405 per year, and formal home care cost was around $27 per hour, or roughly $56,160 annually for 40 hours of in-home care per week. In 2000, home health care expenditures amounted to $32 billion. By 2022, however, this figure had increased to $133 billion (Skaria, 2022).
The most expensive long-term care service in the United States is a nursing home, where the annual cost for a semiprivate room was $104,025 in 2023. With an annual cost of $54,200, a single bedroom in an assisted living facility was cheaper than a nursing home and varied by state.
The average cost of informal care for patients with dementia is more than double the corresponding cost of care for patients without dementia, $83,022 versus $38,272.
As the population ages and families are less able to bear the cost of long-term care, the current system may be unable to meet the growing demand without alternative care programs and sustainable financing (Statista, 2024b).
WHAT IS ALZHEIMER’S DISEASE?
Normal aging involves changes throughout the body, and the brain is not exempt. In normal aging, the volume of the brain decreases each year after age 65, with greatest loss in the frontal and temporal lobes and greater loss of white matter than gray matter in cognitively normal older adults. Cerebral blood flow decreases up to 5%–20%, with deterioration of mechanisms that maintain cerebral blood flow with fluctuation in blood pressure.
Age-related neuronal loss is most prominent in the largest neurons in the cerebellum and cerebral cortex. The hypothalamus, pons, and medulla have modest if any neuron or volume losses with normal aging. Age-related neuron loss is likely due to programmed cell death (apoptosis) rather than inflammation, ischemia, or other mechanisms.
Age also affects neurons that persist, with loss of dendritic tree, shrinkage of processes, and decrease of synapses. Such changes may contribute more to age-related loss of brain volume than the loss of neurons. In some areas, dendritic connections may increase, which may be due to repatterning of the brain invoked to compensate for cellular death. Neurons continue to form new synapses, and new neurons are formed throughout the lifespan, but rates of loss are greater than gains.
Lipofuscin accumulates in certain areas of the brain, particularly the hippocampus and frontal cortex, both of which are associated with memory formation, but the impact of lipofuscin on function is unknown.
Neurofibrillary tangles and amyloid plaques, which are two characteristic lesions of Alzheimer’s disease, occur in certain areas of the brain in normal aging but to a lesser extent than in Alzheimer’s disease. More than 50% of cognitively normal individuals over age 85 have sufficient plaques/tangle burden to make a pathologic diagnosis of Alzheimer’s disease (Taffet, 2023).
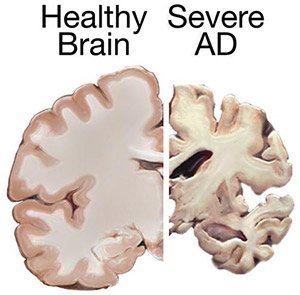
Shrinkage of the brain due to Alzheimer’s disease. (Source: National Institute on Aging/National Institutes of Health.)
Pathophysiology
Alzheimer’s disease is characterized by two abnormalities in the brain: amyloid plaques and neurofibrillary tangles. Neurofibrillary tangles are bundles of twisted filaments found within neurons. These tangles are largely made up of a protein called tau. Amyloid plaques, which are found in the tissue between the nerve cells, are unusual clumps of a protein called beta-amyloid and degenerating bits of neurons and other cells (Stanford Medicine, 2024a).
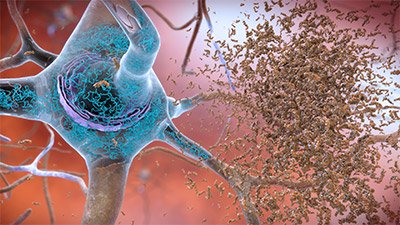
Neurofibrillary tangles and amyloid plaques. (Source: National Institute on Aging/National Institutes of Health.)
These pathologic changes are accompanied by a loss of neurons, particularly cholinergic neurons in the basal forebrain and the cortex. Two prominent pathophysiological hypotheses have been proposed based on these pathological findings.
- The cholinergic hypothesis proposes that the reduced levels of acetylcholine in the brain resulting from neuronal loss play a significant role in AD development. This hypothesis stems from the early loss of cholinergic neurons in AD. Beta-amyloid (also called amyloid beta) is believed to negatively affect cholinergic function by causing cholinergic synaptic loss and impaired acetylcholine release. Anticholinergics also adversely affect memory in older patients clinically.
- The amyloid hypothesis is currently the most widely accepted pathophysiologic mechanism for AD, especially in cases of inherited AD. The amyloid hypothesis suggests that amyloid beta (Aβ) peptide is derived from amyloid precursor protein (APP) through the actions of beta- and gamma-secretase enzymes. Usually, APP is split by either alpha- or beta-secretase, and the tiny fragments formed by them are not toxic to neurons—however, sequential splitting by beta- and then gamma-secretase results in 42-amino-acid peptides (Aβ42). Elevation in levels of Aβ42 leads to aggregation of amyloid that causes neuronal toxicity.
(Kumar et al., 2024)
A 2023 study indicates an additional hypothesis that a form of cell death known as ferroptosis (caused by a buildup of iron in the cells) destroys microglia cells (involved in the brain’s immune response) in cases of Alzheimer’s and vascular dementia. The researchers discovered that microglia degenerate in the white matter of the brain of patients with Alzheimer’s and vascular dementia, and the cascading effect of this degeneration appears to be a mechanism in advancing cognitive decline. The underlying cause of the cycle of decline is likely related to repeated episodes of low blood flow and oxygen delivery to the brain over time due to acute stroke or chronic conditions such as hypertension and diabetes (Robinson, 2023).
Other theories include:
- Tau propagation hypothesis
- Inflammation hypothesis
- Oxidative stress hypothesis
- Mitochondrial hypothesis
- Infectious hypothesis
- Calcium hypostasis hypothesis
- Metal ion hypothesis
- Ion channel cell hypothesis
- Autoimmune hypothesis
- Epigenetic hypothesis
(Mehta & Mehta, 2023)
Etiology and Risk Factors of Alzheimer’s Disease
Alzheimer’s is a complex disease with no single, clear-cut etiology and therefore no sure means of prevention or “silver bullet” cure or treatment. Scientists understand that for most people Alzheimer’s is an ecologic disease caused by genetics and the interaction of genes with other internal and external factors over many years, leading to changes in brain structure and function. This means that genetics plays an important role, together with how genes are affected by external factors such as environment and lifestyle (epigenetics), some of which are modifiable and some of which are not.
GENETIC RISK FACTORS
Variations in genes—even small changes—can affect the likelihood of a person developing Alzheimer’s. In most cases, AD does not have a single genetic cause. Instead, it can be influenced by multiple genes in combination with lifestyle and environmental factors. A person may carry more than one genetic variant or group of variants that can either increase or reduce the risk of Alzheimer’s.
People who develop AD do not always have a history of the disease in their families. Those with a parent or sibling with the disease, however, have a higher risk of developing it than those who do not have a close relative with the disease.
Genes Linked to Alzheimer’s Disease
Genetic variants that affect AD risk include the apolipoprotein (APOE) gene. The APOE E4 allele increases risk for Alzheimer’s and is associated with an earlier age of disease onset. Some people with this allele, however, never develop the disease.
Rare variants in three genes are known to cause Alzheimer’s:
- Amyloid precursor protein (APP) on chromosome 21
- Presenilin 1 (PSEN1) on chromosome 14
- Presenilin 2 (PSEN2) on chromosome 1
A child whose biologic parent carries a genetic variant for one of these three genes has a 50/50 chance of inheriting that altered version of the gene.
When Alzheimer’s develops before age 65, it is known as early-onset Alzheimer’s or sometimes younger-onset Alzheimer’s. Less than 10% of all people with Alzheimer’s develop symptoms this early. Of those who do, 10% to 15% can be attributed to changes in APP, PSEN1, and PSEN2 (NIA, 2024a).
Down Syndrome and Alzheimer’s
People with Down syndrome have an increased risk of developing Alzheimer’s, and this is believed to be related to trisomy 21, which includes the gene that encodes for the production of APP, which in people with Alzheimer’s is cut into beta-amyloid fragments that accumulate into plaques. Amyloid accumulation is seen in almost all adults over age 40 with Down syndrome. Despite these brain changes, however, not everyone with Down syndrome develops Alzheimer’s symptoms.
People with Down syndrome are also very likely to experience severe issues related to their heart, which places them at increased risk for early-onset dementia. Many people with Down syndrome are diagnosed with AD in their 50s, but it is not uncommon for symptoms to occur in the late 40s (CDC, 2022).
TREM2 and Chronic Inflammation in the Brain
The triggering receptor expressed on myeloid cells 2 (TREM2), a transmembrane receptor abundantly expressed on microglia, has been identified as one of the risk factors of AD.
Studies have demonstrated that the gene TREM2 R47H variant is a risk factor for AD. TREM2 promotes the seeding and spreading of tau aggregates in nerve plaques. TREM2 deficiency prevents microglia from aggregating around beta-amyloid deposits, causing senile plaques to spread more, thus increasing neuronal damage. TREM2 can not only influence microglial functions in amyloid and tau pathologies, but also participates in inflammatory responses and metabolism (Huang et al., 2023).
EPIGENETIC RISK FACTORS
Epigenetics (how certain genes are turned on and off) influences many complications at cellular and molecular levels, including Alzheimer’s disease.
Growing evidence points to epigenetic changes as playing a pivotal role in the pathogenesis of the disease. The dynamic interplay between genetic and environmental factors influences the epigenetic landscape in AD, altering gene expression patterns, key pathologic events associated with disease pathogenesis. To this end, epigenetic alterations not only impact the expression of genes implicated in AD pathogenesis but also contribute to the dysregulation of crucial cellular processes, including synaptic plasticity, neuroinflammation, and oxidative stress.
In Alzheimer’s disease, the epigenetic mechanisms include DNA methylation, histone modification, and noncoding RNA, which alter gene expression at the transcription level by upregulation, downregulation, or silencing of genes that have a substantial impact on the progression and pathways linked to Alzheimer’s disease (DePlano et al., 2024).
Chronic Stress and Depression
Depression in known to be a risk factor for dementia, but Alzheimer’s may also be part of the disease itself. The connection is complicated, and how the two conditions are linked is unclear. Further research is needed to evaluate whether the link may be biologic; a result of behaviors associated with depression, such as social isolation and other changes in key health behaviors; or some combination of these mechanisms.
It is not fully understood whether the length of depression, the severity of depression, or the age at which someone experiences depression affects their dementia risk. Research shows that depression can increase risk of dementia whether it occurs in early adulthood, midlife, or later life. It is important to note that, while there is a connection, not everyone who has depression will go on to develop dementia and not everyone with dementia will develop depression (Alzheimer’s Society, 2024d; Penn Medicine, 2023).
Chronic stress and depression are potential risk factors for mild cognitive impairment and dementia, including Alzheimer’s disease. A diagnosis of depression has been found to be associated with an approximately twofold increased risk for later Alzheimer’s disease. The risk increases further in patients who also experience chronic stress. This suggests that chronic stress and depression may be independent risk factors for dementia and that together they may have an additive effect on the risk for later dementia (Wallensten et al., 2023).
Diet
The risk of AD is known to be increased by diets high in table sugar (sucrose) or high-fructose corn syrup, high-glycemic-index carbohydrates, salty foods, and alcohol, as well as processed meats rich in umami flavor (savory, meaty, brothy, smoky, or earthy).
Results from a 2023 study suggest that Alzheimer’s disease could be a maladaptation of an evolutionary survival pathway mediated by intracerebral fructose and uric acid metabolism. High levels of fructose, particularly those derived from added sugars such as sucrose and high-fructose corn syrup, could alter brain metabolism and cause degeneration of brain regions associated with Alzheimer’s disease. A prolonged decline in metabolism in higher cognitive functions may cause degeneration of these regions and lead to the cognitive decline observed in Alzheimer’s disease (Johnson et al., 2023; Shukla, 2023).
Excess Deregulated Brain Iron
Iron excess has been found to be pivotal in the pathogenesis of Alzheimer’s disease. In patients carrying the APOE4 allele, the increase in iron detected in cerebrospinal fluid (CSF) has been found to be strongly associated with cognitive decline, indicating that iron imbalance can be one of the risk factors for AD. Iron overload and oxidative stress in the brains of people with AD have been associated with the aggregation of beta-amyloid-induced senile plaque deposition and hyperphosphorylated tau proteins that form neurofibrillary tangles in the brain (Levi et al., 2024).
Diabetes Mellitus
Scientists are finding more evidence that links type 2 diabetes with Alzheimer’s disease. Several research studies following large groups over many years suggest that adults with type 2 diabetes have a higher risk of developing Alzheimer’s. One study found that people with high blood sugar levels—such as those linked with type 2 diabetes—had a dramatic increase in beta-amyloid protein, one of the hallmark brain proteins of Alzheimer’s disease. Another study found that those whose onset of type 2 diabetes was at a younger age are at high risk of dementia. People with type 1 diabetes were found to be 93% more likely to develop dementia (Alzheimer’s Association, 2023b).
Cardiovascular Disease
Studies link Alzheimer’s disease with cardiovascular risk factors such as hypertension, inflammation, and dyslipidemia. One third of Alzheimer’s disease–related dementias are attributable to modifiable atherosclerotic cardiovascular disease risk factors, such as hypertension, which promote the accumulation of amyloid, a hallmark pathology in the brains of people with AD.
Atherosclerosis is associated with hypoxia, inflammation, oxidative stress, and the accumulation of advanced glycation end products, all of which are factors that can enhance the deposition and/or reduce clearance of amyloid in the brain. Atherosclerosis could contribute to brain dysfunction and axonal damage by a subtle reduction in microvascular perfusion without causing overt ischemic lesions. In addition, subclinical atherosclerosis in coronary, carotid, and femoral arteries has been linked with dementia and AD incidence.
Arteriosclerosis, marked by measures of pulse wave velocity (among others), is associated with cognitive impairment, and studies suggest that intracranial arteriosclerosis (the hardening of arteries due to calcification) may play a role in the etiology of dementia and Alzheimer’s disease (Saeed et al., 2023).
Hearing Loss
People who develop hearing problems during midlife (ages 40–65) have an increased risk of developing dementia. Even low levels of hearing loss have been associated with increased dementia risk and a decrease in memory and thinking skills. Hearing loss has also been linked to quicker shrinkage of areas of the brain responsible for processing sounds and memories.
Peripheral hearing loss involves reduced abilities of the ears to detect sounds. This increases the person’s risk of developing dementia. Central hearing loss involves problems with processing sounds in the brain that are not correctable with hearing aids. This may be a very early symptom of Alzheimer’s disease, as sound-processing parts of the brain are affected by the disease.
People with hearing problems may also be more likely to withdraw from social situations and become more isolated and depressed over time. Social isolation and depression are both risk factors for dementia.
The use of hearing aids has been shown to reduce the risk of dementia to the level of a person with normal hearing (Alzheimer’s Society, 2024c).
Head Trauma
Certain types of traumatic brain injury (TBI) have been found to increase the risk of developing Alzheimer’s or other dementias years after the injury. Older adults with a history of moderate TBI have a 2.3 times greater risk of developing Alzheimer’s compared to older adults with no history of head injury. Those with a history of severe TBI have a 4.5 times greater risk.
The risk of a dementia diagnosis is highest during the first year following TBI. During this time people are four to six times as likely to develop a dementia diagnosis as those without TBI. One study found that a concussion or TBI can increase the risk of developing dementia even 30 years later.
Ongoing research is aimed at understanding the underlying mechanisms of the connection between TBI, cognitive decline, and dementia in the brain, including the role of potential exacerbating factors (Alzheimer’s Association, 2024f).
Smoking
Smoking is known to increase the risk of vascular problems, such as strokes or small bleeds in the brain, both of which are risk factors for dementia. Toxins in cigarette smoke also cause cell inflammation and oxidative stress, both of which of which have been linked to Alzheimer’s. Smoking may be responsible for 14% of Alzheimer’s cases worldwide. An analysis of 37 different studies suggested that current smokers are 30% more likely to develop dementia and 40% more likely to develop Alzheimer’s. In addition, the study found that smoking 20 cigarettes per day increases the risk of dementia by 34% (ALZRA, 2024).
Vitamin Deficiency
Low levels of vitamin D, vitamin B6, vitamin B12, and folate have been found to increase the risk of dementia. Research suggests that people with very low levels of vitamin D are at higher risk for developing Alzheimer’s disease and other forms of dementia. Vitamin D is known to participate in the clearance of amyloid beta aggregates and may provide neuroprotection against amyloid beta-induced tau hyperphosphorylation. Low levels of serum vitamin D have been associated with a greater risk of dementia and AD (Ghahremani et al., 2023).
Obesity
Obesity (defined as having a body mass index of 30 or above) between the ages of 35 and 65 can increase dementia risk in later life by about 30%. Being overweight but not obese, however, does not carry the same risk. Obesity is also linked to other dementia risk factors. People with obesity are two to three times more likely to have high blood pressure and type 2 diabetes. Obesity is also a risk factor for depression and is associated with social isolation and less physical activity—all of which contribute to the risk of dementia.
The size of a person’s brain begins to decrease naturally as they age. However, research has shown a relationship between BMI and brain size in people around age 60. The higher someone’s BMI, the smaller their brain. It has been suggested that the increased brain shrinkage associated with obesity ages the brain by around 10 years. Research has also shown that the areas of the brain that begin to shrink more in Alzheimer’s disease also shrink in people who are obese.
Obesity can lower a person’s resilience to the damage in the brain that Alzheimer’s disease causes, leading to worse symptoms and faster disease progression. Obesity can also lead to chronic inflammation in the body, causing the overactivation of immune cells in the brain, which leads them to damage the brain’s nerve cells (Alzheimer’s Society, 2024e).
Ophthalmic Comorbidities
Generally, visual impairment has been found to be associated with an increased incidence of dementia. Visual impairment increases dementia risk via several mechanisms similar to hearing impairments, such as increasing cognitive demands, leading to social isolation and physical inactivity, and even causing structural and functional changes in the brain. Several eye disorders, such as cataracts, age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy, are reported to be associated with AD and other dementias.
One study found that moderate-to-severe visual impairment could increase the risk of dementia by two times in the first two years and 1.8 times from two to four years of follow-up, although the association was not significant beyond four years. Studies also concluded that a more severe visual impairment was associated with a higher incidence of dementia (Zheng et al., 2024).
Hormones
Women make up an estimated 65% of people who currently have dementia. It is not fully understood why women are more likely to develop Alzheimer’s disease, the most common cause of dementia. One idea is that it may have to do with the hormone estrogen.
Research has shown that estrogen may help to protect the brain from Alzheimer’s by blocking some of the harmful effects of the amyloid-β protein. Estrogen can also affect the way chemical messengers such as serotonin, acetylcholine, and dopamine are used to send signals throughout the brain, and some Alzheimer’s disease symptoms are linked to these chemical messengers.
Alzheimer’s disease is more common in women after menopause, so it is possible that this protective effect may be lost when estrogen levels are decreased in older women. It is not clear whether hormone replacement therapy (HRT) reduces dementia risk or not, with some studies suggesting that estrogen may reduce dementia risk while others saying it increases the risk (Alzheimer’s Society, 2023a).
SEX
One’s sex plays a role in the development of Alzheimer’s disease and involves both genetics and epigenetics. After advanced age, being female is the second major risk factor for Alzheimer’s. This may be due to differences in biology, such as menstruation, pregnancies, and menopause. It may also be related to traditional differences in gender roles, such as education, work, and lifestyle.
An important factor is that women who have the APOE4 gene variant have greater risk. Nearly two thirds of people with AD have at least one copy of this gene. Although men and women are just as likely to have the gene variant, its effect on dementia risk seems to be greater in women than in men. The reasons for this difference are not fully understood.
The expression of genes that contribute to the development of amyloid and tau pathology shows a female bias, and higher expression of these genes generates high amounts of protein involved in amyloid and tau pathology. Other characteristics, such having defective versions of neural survival factors, may contribute to women being more susceptible to developing AD (Alzheimer’s Society, 2024b; Reed-Geaghan, 2022).
Traumatic brain injury is another dementia risk factor where there may be important differences between the sexes, since women are more vulnerable to concussion and its long-term effects on the brain.
However, the vast majority of experiments and clinical trials carried out by dementia researchers have not been designed to find differences between male and female participants. Most older studies involving mice used only males, and the first phases of clinical trials recruited only male volunteers (Alzheimer’s Society, 2024b).
Possible Preventative Strategies
Currently, there is little definitive evidence about what can stop age-related cognitive decline and the onset of dementia, but more and more research suggests that attaining better brain health and resilience, even later in life, can postpone the critical moment when a change or effect becomes irreversible.
EXERCISE
Regular physical exercise may be a beneficial strategy to lower the risk of Alzheimer’s and vascular dementia. Exercise may directly benefit brain cells by increasing blood and oxygen flow in the brain. Because of its known cardiovascular benefits, a medically approved exercise program is a valuable part of any overall wellness plan.
There are two main types of physical activity—aerobic and strength building. Each type has different benefits, and performing a combination of both types may help to reduce risk of dementia. Most studies report on the effects of aerobic exercise done several times a week and maintained for at least a year. Physical activity, however, can also mean a daily activity such as brisk walking, cleaning, or gardening. One study found that daily physical tasks such as cooking and washing up can reduce the risk of Alzheimer’s disease (Alzheimer’s Association, 2024g; Alzheimer’s Society, 2024f).
DIET
The typical Western diet increases cardiovascular disease risk, possibly contributing to faster brain aging. It is possible that diet affects biologic mechanisms such as oxidative stress and inflammation, both of which underlie Alzheimer’s. Eating a certain diet might increase specific nutrients that may protect the brain through anti-inflammatory and antioxidant properties, by inhibiting beta-amyloid deposits, or by improving cellular metabolism in ways that protect against the disease.
Research has shown, with mixed evidence, that two diets that may hold potential benefits for cognitive health:
- The Mediterranean diet emphasizes fruits, vegetables, whole grains, legumes, fish and other seafood, unsaturated fats such as olive oils, and low amounts of red meat, eggs, and sweets.
- The MIND (Mediterranean–DASH Intervention for Neurodegenerative Delay) diet is a hybrid of the Mediterranean and the DASH (Dietary Approaches to Stop Hypertension) diet. The MIND diet features vegetables, especially green leafy vegetables; berries over other fruit; whole grains; beans and nuts; one or more weekly servings of fish; and olive oil. It also limits servings of red meat, sweets, cheese, butter/margarine, and fast/fried food.
Other studies have shown that a molecule in green tea breaks apart tangles of the protein tau and that older adults who reported eating a daily serving of leafy green vegetables such as spinach or kale showed slower age-related cognitive decline, perhaps due to the neuroprotective effects of certain nutrients. Another recent study in mice found that consuming too much salt increased levels of the protein tau (NIA, 2023b).
SLEEP
Some evidence suggests that a lack of sleep may increase the risk of dementia, but researchers are not sure how sleep and dementia are linked. Does poor sleep increase dementia risk, does dementia lead to poor sleep, or both? One study found that nine or more hours sleep duration was associated with a 50% increase in Alzheimer’s mortality risk when compared to people who slept seven to eight hours, particularly in men. Additional studies are needed to further elucidate the mechanisms (Alzheimer’s Society, 2023b; Scheider et al., 2022).
PREVENTING HEAD TRAUMA
Because there is an association between traumatic head injury and an increased risk for Alzheimer’s dementia, it is important to take the following protective steps:
- Choose a sports program that enforces rules for safety and avoids drills and plays that increase the risk for head impacts.
- Wear a seat belt when driving or riding in a motor vehicle.
- Never drive while under the influence of alcohol or drugs.
- Wear a helmet or appropriate headgear when riding a bike or any other open vehicle and when taking part in any sport activities.
- Have a primary care provider or pharmacist review medications for safety concerns, including prescription medicines, over-the-counter medicines, herbal supplements, and vitamins.
- Have vision checked at least once a year and update eyeglasses if necessary.
- Perform appropriate-level strength and balance exercises.
- Make the home as safe as possible to prevent falls.
(CDC, 2024)
SOCIAL CONNECTIONS
It is difficult to know how much social isolation by itself contributes to the risk for dementia. Isolation might also occur as a consequence of dementia, and it is also tied to other risk factors, including physical inactivity and depression.
Social isolation can increase the risk of dementia by about 60%. Studies show that lifelong single people are more likely to develop dementia than those who are married. Widowed people are slightly more likely to develop dementia. Social isolation is less common in those who are married, as married people often have more social contact with others than single people.
Lonely people are more likely to drink heavily, smoke, not exercise, be overweight, and have heart problems, all of which increase the risk of dementia.
Engaging in social activities may help the brain’s ability to cope with disease, relieve stress, and improve mood. Examples can include:
- Adult education or learning
- Arts and crafts (especially in groups)
- Playing a musical instrument or singing
- Volunteering
(Alzheimer’s Society, 2024g)
VACCINATIONS
Several vaccines are associated with reduced risk of Alzheimer’s disease in adults ages 65 and older. Some experts theorize that infection plays a role in developing Alzheimer’s disease and that vaccinations may help stave off these infections. Others say it is possible that vaccination may reduce an immune system function that attacks amyloid plaque as an invader.
Studies have found that patients who received at least one influenza vaccine during the follow-up period of four years were 40% less likely to develop Alzheimer’s compared to those who did not get the vaccine; that those who received an annual flu shot had the lowest rate of the disease; and that regular vaccination against tetanus, diphtheria, whooping cough (pertussis), shingles, and pneumococcal pneumonia has been associated with a 25%–30% percent reduced risk of Alzheimer’s disease in patients ages 54 and older.
These findings suggest that vaccination has a more general effect on the immune system that may reduce the risk for developing AD (Barkley, 2023; Cimons, 202; Hsu, 2023).
BRAIN TRAINING
Brain training can improve memory and thinking, but there is no strong evidence that brain training activities will reduce a person’s risk of developing dementia. People who regularly do intellectual activities throughout life have stronger thinking abilities, which give them skills that may protect them against losses that can occur through aging and disease (Alzheimer’s Society, 2024h).
ALZHEIMER’S DISEASE SIGNS AND SYMPTOMS
The cardinal symptoms of Alzheimer’s disease include:
- Memory impairment
- Executive function and judgment/problem-solving impairment
- Behavioral and psychological symptoms
Memory Impairment
Early in the course of Alzheimer’s, individuals are usually aware of their memory deficit and may make notes to remember important things. Sooner or later the memory deficit is such that they may forget to check their notes. Later they may become frightened and apprehensive about their memory problems, causing them to feel depressed and discouraged. As the disease progresses, individuals lose insight into their memory deficit and are no longer aware of it. It is at this point that they require protection to remain safe.
The following are early signs and symptoms of Alzheimer’s disease memory impairment compared to typical age-related changes.
| Memory Impairment with Alzheimer’s | Typical Age-Related Memory Changes |
|---|---|
| (Alzheimer’s Association, 2024h) | |
|
Memory loss that disrupts daily life:
|
Developing very specific ways of doing things and becoming irritable when the routine in disrupted |
Challenges in planning or solving problems:
|
Making occasional errors when managing finances or household bills |
|
Difficulty completing familiar tasks, such as:
|
Occasionally needing help to use household devices (e.g., microwave setting) or other technical or mechanical device |
|
Confusion about time and place:
|
Getting confused about the day of the week but figuring it out later |
|
Trouble understanding visual images and spatial relationships:
|
Vision changes related to cataracts or other age-related changes or diseases |
|
New problems with words in speaking or writing:
|
Sometimes having trouble finding the right word |
|
Misplacing things and losing the ability to retrace steps:
|
Misplacing things from time to time and retracing steps to find them |
|
Decreased or poor judgment:
|
Making a bad decision or mistake once in a while (e.g., neglecting to change the oil in the car) |
|
Withdrawal from work or social activities:
|
Sometimes feeling uninterested in family or social obligations |
|
Changes in mood and personality:
|
Developing very specific ways of doing things and becoming irritable when a routine is disrupted |
HOW MEMORIES ARE MADE
Memory is a multifaceted cognitive process involving different stages: encoding, consolidation, retrieving, and reconsolidation.
Encoding involves acquiring and processing information that is transformed into a neuronal representation suitable for storage. The information can be acquired through various channels, such as visual, auditory, olfactory, or tactile inputs.
The acquired sensory stimuli are converted into a format the brain can process and retain. Different factors such as attention, emotional significance, and repetition can influence the encoding process and determine the strength and durability of the resulting memory.
Consolidation includes the stabilization and integration of memory into long-term storage to increase resistance to interference and decay.
Retrieving is the process of retrieving stored information. One critical factor involved in retrieval is the type of hints or cues in the environment (McDermott & Roediger, 2024).
TYPES OF MEMORY AND ASSOCIATED AD SYMPTOMS
Alzheimer’s disease is a progressive neurodegenerative disease marked by deficits in episodic memory, working memory, and executive function.
Memory is a large part of a person’s identity, and there are different types. Each type uses a different network in the brain, and therefore, one type can be affected by disease or injury while another type functions normally. Memory systems are divided into two broad categories:
- Declarative memory allows us to explicitly recall or recognize past events as facts (i.e., “knowing what”). Recalling information from declarative memory involves some degree of conscious effort; information is consciously brought to mind and “declared.”
- Nondeclarative memory is different in that it is accessed without conscious thought. It can be triggered implicitly and is expressed through performance, such as the skill of riding a bike (i.e., “knowing how”). Another form of nondeclarative memory is the ability to improve one’s perception of the surrounding world through practice (called perceptual memory).
(LaFee, 2021)
Episodic Memory
Episodic memories are what most people think of as “memory” and include information about recent or past events and experiences. Typically, Alzheimer’s disease is associated with a decline in episodic memory. People with AD complain that they can’t remember events they’ve experienced, conversations they’ve had, or things they’ve done.
The hippocampus and surrounding structures in the temporal lobe are important in episodic memory and are part of an important network called the default mode network and made up of several brain areas including frontal and parietal regions. This network has been implicated in episodic memory functioning (UCSF, 2024a).
A person with dementia may experience “time shifting.” This is when a person’s current experience is that they are living at an earlier time in their life (Alzheimer’s Society, 2023c). This explains why individuals with AD eventually “live in the past,” such as wanting to milk the cows even though they haven’t lived on a farm for over 20 years, wanting to go to work even though they retired 30 years ago, etc. It also explains why they’re able to remember events many decades old but unable to remember what happened that morning.
Semantic Memory
Like episodic memory, semantic memory is declarative and refers to a person’s general knowledge, including common knowledge of facts about the world. It is also used to remember familiar faces or objects. Problems with semantic memory can include:
- Anomia, or the inability to find the right word. At first the person with Alzheimer’s is aware of this and may make up for it by using sentences to describe an object they cannot name. As the condition worsens, anomia comes to include common objects such as an eating utensil or pen.
- Aphasia, or difficulty with and eventual loss of the ability to speak or understand spoken, written, or sign language. In the advanced stage of the disease, speech becomes unintelligible, and eventually the person becomes mute.
- Agnosia, or loss of the ability to recognize what objects are and what they are used for. This may involve failing to recognize who people are. Agnosia can be visual, auditory, or tactile, but visual is the most common form.
(Blanchard, 2024)
Procedural Memory
Procedural memory is a form of nondeclarative memory, a type of long-term memory that stores information related to motor skills, habits, and actions that allow individuals to perform tasks automatically and without conscious effort. It involves learning and retention of procedures, routines, and how to execute specific actions.
The loss of procedural memory can result in difficulties carrying out routine activities such as dressing, bathing, and cooking.
Apraxia is one of the most common deficits in procedural memory observed in patients with Alzheimer’s. It is a disorder of “how” and “when” to correctly perform meaningful and purposeful actions. A person with apraxia is unable to put together the correct muscle movements, which can also lead to problems with speech. These may include:
- Ideomotor apraxia: the inability to act out a movement, such as turning a doorknob, even though one can describe how it is done
- Ideational apraxia: the inability to conceptualize and perform tasks that involve multiple sequential movements (such as putting on socks before shoes)
- Limb-kinetic apraxia: the inability to make precise, coordinated finger and hand movements (such as buttoning a shirt)
- Buccofacial/orofacial apraxia: the inability to perform facial movements on demand (such as licking lips, whistling, or winking)
- Verbal apraxia (apraxia of speech): difficulty coordinating mouth movements to produce speech
- Constructional apraxia: the inability to copy or draw objects or symbols
- Oculomotor apraxia: the inability to move the eyes on demand
(Gasnik, 2024; Simply Psychology, 2022)
Visuospatial Memory
Visuospatial memory gives one the ability to navigate in the environment and to identify, integrate, and analyze space and visual form, details, structure, and spatial relations in several dimensions. It requires the formation, storing, and retrieval of mental maps. In persons with Alzheimer’s, loss of this ability results in getting lost in familiar surroundings, wandering, and losing the ability to live independently (Kim & Lee, 2021).
Visuospatial ability examples include:
- Using a map to get from one place to another rather than relying on directions
- Walking through doorways rather than bumping into the door frames
- Driving or crossing the road; judging vehicle distance and speed accurately
- Looking at something and picking it up; guiding the hand accurately to the item wanted
A person with visuospatial problems finds it difficult to interpret what is being seen and to act appropriately.
Working Memory
Working memory can be defined as the ability of the brain to keep a limited amount of information available long enough to use it. Working memory helps process thoughts and plans as well as carry out ideas. It is the short-term memory that combines strategies and knowledge from the long-term memory bank to assist in making a decision or calculation. Working memory has been connected to executive functioning, which is often affected in the earlier stages of Alzheimer’s disease (Heerema, 2022a).
Executive Function, Judgment, and Problem-Solving Impairment in AD
Executive functioning refers to high-level cognitive skills required for control and coordination of other cognitive abilities and behaviors. Executive functions can be divided into organizational and regulatory abilities.
Organizational abilities are techniques used by individuals to facilitate the efficiency of future-oriented learning, problem-solving, and task completion. Organization requires the integration of several elements to reach a planned goal. These include:
- Attention: the capacity to be paying attention to a situation or task in spite of distractions, fatigue, or boredom
- Planning: the ability to create a mental roadmap to reach a goal or to complete a task
- Sequencing: the ability to perceive and execute a set of actions that follow a particular order
- Problem-solving: the capacity to identify and describe a problem, and generate solutions to fix it
- Working memory: the ability to hold information in mind while performing complex tasks
- Cognitive flexibility: the ability to revise plans in the face of obstacles, setbacks, new information, or mistakes
- Abstract thinking: the ability to think about objects, principles, and ideas that are not physically present
- Rule acquisition: how individuals acquire skill in applying problem-solving rules
- Selecting relevant sensory information: how an individual analyzes incoming sensory information in order to form visually guided motor decisions
Regulatory abilities involve evaluating available information and modulating a response to the environment. These include:
- Initiation of action: the ability to begin a task in a timely fashion
- Self-control: the ability to manage emotions in order to achieve goals, complete tasks, or control and direct behavior
- Emotional regulation: the ability to keep one’s emotions under control
- Monitoring of internal and external stimuli: the ability to monitor and change behavior as needed, plan future behavior when faced with new situations, and anticipate outcomes to changing situations
- Initiating and inhibiting context-specific behavior: the ability to inhibit or control impulsive (or automatic) responses and create responses by using attention and reasoning
- Moral reasoning: the logical process of determining whether an action is right or wrong
- Decision-making: the ability to be proficient in making choices between two or more alternatives
(UCSF, 2024b)
Damage to the executive system often leads to:
- Difficulty organizing
- Difficulty in planning and initiation
- Inability to multitask
- Difficulty with verbal fluency
- Trouble planning for the future
- Difficulty processing, storing, and/or retrieving information
- Mood swings
- Lack of concern for people and animals
- Loss of interest in activities
- Socially inappropriate behavior
- Inability to learn from consequences elating to past actions
- Difficulty with abstract concepts (the ability to make the leap from the symbolic to the real world)
- Unawareness or denial that their behavior is a problem
(UCSF, 2024b)
Early in Alzheimer’s, impairment of executive function can range from subtle to prominent. In many cases, the person underreports these symptoms, and an informant interview is critical. Family and coworkers may notice that the person is less organized or less motivated and that their ability to multitask is often impaired. In addition, the person develops poor insight and reduced ability for abstract reasoning. As Alzheimer’s progresses, the person becomes unable to complete tasks.
The patient with Alzheimer’s often has anosognosia, which is a reduced insight into one’s neurologic deficits. Many patients underestimate their deficits or try to offer explanations or alibis for them when they are pointed out by others. It is often a family member and not the patient who brings cognitive impairment to the attention of healthcare professionals. Loss of insight may be associated with behavioral disturbance. Those with relatively preserved insight are more likely to be depressed. Those with more impaired insight are likely to be agitated, be disinhibited, and exhibit psychotic features. Lack of insight can present problems with safety when patients attempt tasks they are no longer able to perform, such as driving (Wolk & Dickerson, 2024).
Psychological and Behavioral Symptoms in AD
Psychological and behavioral symptoms are common in Alzheimer’s disease, in particular during the middle and late stages of the illness. Psychological and behavioral symptoms affect up to 90% of patients diagnosed with dementia during the course of their illness. Patients with psychological and behavioral symptoms experience emotional distress, diminished quality of life, greater functional impairment, more frequent hospitalizations, increased risk of abuse and neglect, and decreased survival. Caregivers experience increased burden of stress and depression.
Some common psychological and behavioral changes are described in the table below:
| Category | Examples |
|---|---|
| (UCSF, 2024c) | |
| Apathy and indifference |
|
| Depression and dysphoria |
|
| Euphoria and elation |
|
| Anxiety |
|
| Irritability and lability |
|
| Inappropriate behaviors |
|
| Eating disorders |
|
| Sleep disturbances |
|
| Repetitive motor behaviors |
|
| Psychosis (during late-stage AD) |
|
Clinical Stages of Alzheimer’s Disease and Related Signs and Symptoms
Patients with Alzheimer’s disease go through different stages as the disease progresses and exhibit different signs and symptoms during each stage. The rate of progression of Alzheimer’s disease varies widely. On average, people with Alzheimer’s live between 3 and 11 years after diagnosis, but some survive 20 years or more.
Alzheimer’s disease has been classified into either three, four, five, or seven stages used for determining the level of care the person requires and for comparing groups of such patients with one another. These classifications are somewhat arbitrary, and there is a great deal of overlap among the various stages. One of the most commonly used classifications divides the disease process into five stages.
STAGE 1: PRECLINICAL ALZHEIMER’S DISEASE
Alzheimer’s disease brain changes begin long before any symptoms become apparent. This stage is called preclinical Alzheimer’s disease. During this stage of the disease there are no noticeable symptoms either to the person with the disease or those around them. This stage can last for years, and even decades, before symptoms begin to appear and a diagnosis is made. This stage is usually identified only in research settings.
STAGE 2: MILD COGNITIVE IMPAIRMENT
During this stage, minor changes in memory and thinking ability develop that are not significant enough to affect work or relationships. The person may have memory lapses when dealing with information that is usually easily remembered, such as conversations, recent events, or appointments. The person in this stage may also have trouble judging time required for completing a task and judging correctly the number of sequences of steps needed to complete a task. Making good decisions may become harder for people during this stage.
Other problems during this stage may include:
- Remembering names
- Recalling recent events
- Remembering where one put a valuable object
- Making plans
- Staying organized
- Managing money
- Remembering material that was just read
- Planning
The person may be aware of memory lapses, and their friends, family, or neighbors may also notice these difficulties.
STAGE 3: MILD DEMENTIA DUE TO ALZHEIMER’S DISEASE
Alzheimer’s disease is often diagnosed in this stage when it is clear to family and healthcare professionals that a person is having significant problems with memory and thinking that impact daily functioning. During this stage, the person may experience:
- Memory loss for recent events
- Difficulty with problem-solving, complex tasks, and sound judgment
- Changes in personality
- Difficulty organizing and expressing thoughts
- Misplacing belongings
- Getting lost
STAGE 4: MODERATE DEMENTIA DUE TO ALZHEIMER’S DISEASE
During this stage, which lasts from 2 to 10 years and is the longest stage, the person becomes more confused and forgetful and begins to require some assistance with daily activities and self-care. The person may experience:
- Increasing trouble planning complicated activities (e.g., preparing a dinner)
- Trouble remembering remote events as well as recent memories
- Problems learning new things
- Trouble remembering their own name
- Being unable to recall information about themselves, such as their address or phone number
- Problems with reading, writing, and working with numbers
As the disease progresses, the person may:
- Know that some people are familiar but cannot remember their names
- Forget the names of a spouse or child
- Lose track of time and place
- Need help with daily self-care activities
- Become moody or withdrawn
- Have personality changes
- Be restless, agitated, anxious, or tearful, especially in late afternoon or at night
- Become aggressive
- Experience sleep problems
- Wander away from home
- Develop psychotic symptoms including hallucinations, paranoia, or delusions
- Lose impulse control
STAGE 5: SEVERE DEMENTIA DUE TO ALZHEIMER’S DISEASE
During this stage of the disease mental functioning continues to decline, and the person loses the ability to communicate coherently. Severe impairment of all cognitive functions occurs, and at this point the person requires total assistance with personal care. As the disease continues to progress the person may remain in bed most or all of the time as the body begins to shut down, and the following may occur:
- Loss of physical abilities, including walking, sitting, and eating
- Inability to hold the head up without support
- Muscles becoming rigid and reflexing abnormally
- Ability to say some words or phrases but inability to have a conversation
- Becoming unaware of recent experiences and surroundings
- Needing help with all activities all of the time
- Becoming unable to recognize immediate family members
- Experiencing seizures
- Increased susceptibility to infection, especially pneumonia
- Sleeping an increased amount
- Losing bladder and bowel control
- Having impaired swallowing (which can lead to aspiration pneumonia, the most common cause of death in persons with Alzheimer’s disease)
(Mayo Clinic, 2023; Johns Hopkins Medicine, 2021a)
AREAS OF THE BRAIN AFFECTED DURING THE STAGES OF ALZHEIMER’S DISEASE
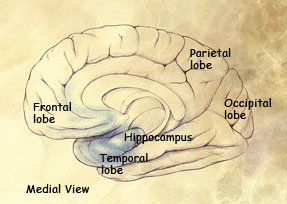
Preclinical stage: Areas of early damage to the hippocampus and portions of the frontal lobe (in blue).
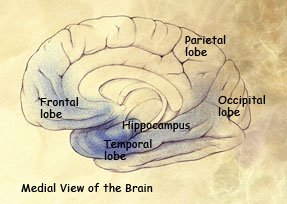
Mild to moderate stages: Spread of damage forward into the frontal lobe and backward into the temporal lobe.
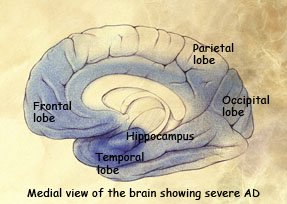
Severe stage: Extensive damage to areas during the final stage of Alzheimer’s disease.
(Source: National Institute on Aging.)
DIAGNOSING ALZHEIMER’S DISEASE
Clinical diagnosis of Alzheimer’s disease is usually made during the early stage, when the person appears to be physically healthy but is having increasing difficulty making sense of the environment. The affected person and the family may mistake early signs of Alzheimer’s for normal age-related changes. Deciding to seek diagnostic testing can be a major hurdle for the person and the family. Admitting that there may be the possibility of a diagnosis of Alzheimer’s disease can be difficult to accept.
There is no single test that can diagnose Alzheimer’s disease. Various approaches and tools are used to assist in making a diagnosis. Diagnosis is made using the following tools:
- Patient medical history
- Physical examination
- Neurologic examination
- Mental cognitive status tests
- Diagnostic tests (to rule out other health issues that can cause similar symptoms to dementia)
- Brain imaging
(Alzheimer’s Association, 2024i)
Patient Medical History
The patient medical history helps to assess past and current health status and includes:
- Patient age and gender
- Chief complaint
- History of the current complaint
- Past medical history
- Current health status
- Psychosocial history such as marital status, living conditions, employment, sexual history, significant life events, diet, nutrition, and use of alcohol or other drugs
- Family medical history, including Alzheimer’s disease or other dementias
- Review of systems to ask questions about current symptoms not included in the chief complaint
- Mood assessment to detect depression or other mood disorders that can cause memory problems, apathy, and other symptoms that can overlap with dementia
- Psychiatric history and history of cognitive and behavioral changes
- Review of all medications
(Alzheimer’s Association, 2024i)
Physical and Neurologic Examinations
The physical and neurologic examinations enable the clinician to assess the overall physical and neurologic condition of the patient and provide more information about the current problem, helping to determine an appropriate plan of treatment. The physical exam may be a complete head-to-toe exam or a more focused examination, depending on the chief complaint. It generally includes:
- Overall appearance
- Vital signs
- Heart and lungs
- Head and neck
- Abdominal exam
- Extremities
- Blood test (see “Diagnostic Testing” below)
- Urine test (to detect formic acid levels, a sensitive biomarker for early-stage Alzheimer’s disease)
(News-Medical.Net. 2024)
The neurologic examination involves evaluating the person for problems that may suggest brain disorders other than Alzheimer’s, which could include Parkinson’s disease, brain tumors, or buildup of fluid in the brain. Family members may be asked to provide input about changes in the person’s thinking skills and behavior. The exam includes:
- Cranial nerve testing
- Reflex testing
- Coordination
- Motor function and balance
- Gait
- Speech
- Muscle tone and strength
- Eye movement
- Sensation
The neurologic exam may also include a fluorodeoxyglucose (FDG) PET imaging study (Alzheimer’s Association, 2024i; Mayo Clinic, 2024).
Mental Cognitive Status Testing
The mental status examination can be divided into the broad categories of:
- Appearance
- Behavior
- Motor activity
- Speech
- Mood
- Affect
- Thought process
- Thought content
- Perceptual disturbances
- Cognition
- Insight
- Judgment
(Voss & Das, 2024)
Many screening tools are available, and the following are particularly useful.
MINI-MENTAL STATUS EXAM (MMSE)
The Mini-Mental State Exam is the most popular and well known of mental status screening tests. It assesses multiple cognitive domains, particularly memory and language, which may be most relevant to dementia due to Alzheimer’s disease. The MMSE can be performed in a relatively short time period (5–10 minutes) and is most sensitive to patients at the moderate stage of Alzheimer’s dementia. It covers a wide range of functions, including memory, attention, orientation, and overall executive function. The tool consists of brief questions and simple tasks scored on a 30-point scale. A score below 24 is considered indicative of dementia.
Advantages of the MMSE include that it estimates the stage and severity of dementia for someone with the disease and that it can show changes over time. In addition, it is easy to administer and requires no special equipment or training.
The disadvantages of the MMSE include that it is less reliable. An educated person with dementia, for instance, might be able to score above 24. It also is not sensitive to mild cognitive impairment or early dementia. The MMSE requires a certain level of education in the patient; someone below an eighth-grade level of education should not take the Mini-Mental State Exam because low educational experience can lead to a misdiagnosis (Dementia Care Central, 2024).
MMSE QUESTIONS AND SCORING
During the MMSE, a health professional asks the patient the following questions or instructs the patient to perform a task:
- What is the date today? (3 points)
- What is the season? (1 point)
- What day of the week is it? (1 point)
- What town, state, and country are we in? (3 points)
- What is the name of this place? (1 point)
- What floor of the building are we on? (1 point)
- I am going to name three objects. After I have said them, repeat them back to me. Remember what they are because I will ask you to name them again in a few minutes: apple, table, penny. (3 points)
- I am going to spell a word forwards and I want you to spell it backwards. The word is W-O-R-L-D. (5 points)
- What are the three objects I asked you to remember a few moments ago? (3 points)
- What is this called (showing the patient a watch)? (1 point)
- What is this called (showing the patient a pencil)? (1 point)
- Please repeat the following: “No ifs, ands, or buts.” (1 point)
- Please read the following and do what it says, handing the patient a card that says, “Please close your eyes.” (1 point)
- Please write a sentence. (1 point)
- Please take this piece of paper in your right hand, fold it in half with both hands, and put it in your lap. (3 points)
- Please copy this drawing (showing the patient a drawing of two overlapping pentagons). (1 point)
The maximum MMSE score is 30 points. A score of 20–24 suggests mild dementia, 13–20 suggests moderate dementia, and <12 indicates severe dementia. On average, the MMSE score of a person with Alzheimer’s declines about 2 to 4 points each year (Dementia Care Central, 2024).
MONTREAL COGNITIVE ASSESSMENT (MoCA)
The Montreal Cognitive Assessment is an in-office test that takes about 10 minutes to complete. It is a 30-point test that assesses seven domains of cognitive function with a total of 11 different exercises and tasks. A score of 26 or higher is considered normal. The seven domains include:
- Executive and visuospatial function
- Naming
- Attention
- Language
- Abstraction
- Delayed recall
- Orientation
Advantages of the MoCA include its simplicity and quickness to administer. On the other hand, training is required to properly administer the test, and the patient’s education level can influence the results (Rosenzweig, 2024).
Other cognitive, functional, and behavioral tests include:
- Ascertain Dementia 8 (AD8)
- Functional Activities Question (FAQ)
- Mini-Cog
- Neuropsychiatric Inventory Questionnaire (NPI-Q)
COMPUTERIZED TESTS
The U.S. Food and Drug Administration has granted permission for several digital cognitive testing tools to be marketed. These include:
- Automated Neuropsychological Assessment Metrics (ANAM)
- Cambridge Neuropsychological Test Automated Battery (CANTAB Mobile)
- CognICA
- Cognigram
- Cognivue
The FDA has also granted permission to market the medical device Cognision, which is a headset with electrodes that are affixed to the scalp to measure electrical activity produced by the brain in order to measure cognitive processes.
One advantage of using these tools is that the tests are given exactly the same way each time. Digital platforms are also well-known for their accessibility. Likewise, the possibility of modifying the contrast, the size or color of the text, the volume, or the response requirements of people with reduced mobility or motor difficulties represent altogether a clear-cut advantage of digital testing.
(Alzheimer’s Association, 2024i)
INFORMANT QUESTIONNAIRES
Informant- or caregiver-completed questionnaires can also be employed to assess a patient for cognitive impairment. These include asking an informant who knows the patient well to answer a series of questions about the patient’s memory and other cognitive functions. Three such questionnaires are:
- Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), available in multiple languages
- AD8 Dementia Screening Interview, an eight-question interview used to distinguish between normal signs of aging and mild dementia
- General Practitioner Assessment of Cognition (GPCOG), available in multiple languages
(Alzheimer’s Association, 2024i)
MEDICARE ANNUAL WELLNESS VISIT
Medicare’s Annual Wellness Visit (AWV) benefit requires, among other things, an assessment to detect cognitive impairments. Along with routine measurements and health risk assessment, during this visit functional ability and level of safety are assessed, as well as ability to perform activities of daily living. Screening is included for cognitive impairment and depression. The cognitive assessment includes a detailed history and patient exam. At this time there should be an independent historian present for the assessments, such as a spouse, guardian, or other individual who can provide the patient’s history when the patient is unable to do so. The cognitive assessment includes a detailed history and patient exam.
Diagnostic Testing
Laboratory tests are performed to rule out other potentially reversible forms of cognitive impairment.
| Test | Associated With |
|---|---|
| (Lakhan, 2024; Stanford Medicine, 2024b) | |
| Folate level | Folate deficiency |
| Cobalamin (vitamin B12) | Hematologic disease |
| Thyroid-stimulating hormone (TSH) and T4 | Thyroid disease |
| Complete blood count (CBC) | Anemia, infection |
| Electrolytes | Renal disease, dehydration |
| Glucose level | Diabetes |
| Urinalysis, microscopy and culture | Urinary tract infection |
| Liver enzymes | Hepatic disease |
| C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) | Inflammatory processes |
| HIV antibody | HIV/AIDS |
| Rapid plasma reagin (RPR), venereal disease research laboratory (VDRL) | Syphilis |
| Toxicology screening | Illicit drug use, alcohol use |
| Paraneoplastic antibodies | Autoimmune encephalitis |
| Less Common Tests | Purpose |
| Cerebrospinal fluid (CSF) biomarkers: beta-amyloid, total tau protein, phosphorylated tau, Lumipulse, and Elecsys | Confirm or rule out Alzheimer’s disease |
| APOE genotype used primarily in research settings | Confirm or rule out probable Alzheimer’s disease |
| PSEN1, PSEN2, and APP | Genetic mutations |
BLOOD TEST FOR ALZHEIMER’S DISEASE
A new blood test specific for diagnosing Alzheimer’s is the PrecivityAD2. It is a biomarker that detects an abnormal version of the tau protein found in neurons affected by Alzheimer’s. Tiny amounts of this protein make their way out of the brain cells and into the bloodstream. Across test participants, the blood test predicted a diagnosis of Alzheimer’s disease with 88% to 92% accuracy. The test measures the ratio of two types of amyloid beta as well as the proportion of a specific type of tau call p-tau217 (Hamilton, 2024; Stanford Medicine, 2024b).
ELECTROENCEPHALOGRAM (EEG)
A recent study indicates that EEG can be used as a useful screening tool for the diagnosis and disease progression evaluation of mild cognitive impairment and Alzheimer’s disease. The most prominent effects of AD-linked neurodegeneration on EEG metrics were localized at parieto-occipital regions. The combination of EEG + CSF + APOE measures achieved the best performance for all targets of prediction (Jiao et al., 2023).
Imaging Studies
Imaging studies are particularly important for ruling out treatable causes of progressive cognitive decline. Alzheimer’s results from the loss of brain cells over time, referred to as degeneration, which may appear in a variety of ways in brain scans. Scans are not used to diagnose Alzheimer’s dementia because there is overlap in what is considered typical age-related change in the brain and change related to Alzheimer’s (Mayo Clinic, 2024).
STRUCTURAL IMAGING
Structural imaging examines the physical structure of the brain by measuring brain tissue volume and identifying areas of atrophy.
Magnetic resonance imaging (MRI) and computed tomography (CT) scans are the structural imaging techniques most commonly used to rule out other conditions that may cause symptoms similar to Alzheimer’s. Structural imaging can reveal tumors, evidence of small or large strokes, damage from severe head trauma, or a buildup of fluid in the brain. In some circumstances, brain imaging tools may be used to determine if the person has high levels of beta-amyloid. Normal levels would suggest Alzheimer’s is not the cause of dementia.
Positron emission tomography (PET) uses a radioactive substance known as a tracer to detect substances in the body. PET scans have recently been developed that detect clusters of amyloid proteins or tau. However, at this time these types of PET scans are typically used in a research setting (Mayo Clinic, 2024.)
FUNCTIONAL NEUROIMAGING
Functional imaging examines how the brain is working by observing blood flow activity in different regions.
Functional neuroimaging enables in vivo (within a living organism) examination of how the brain functions. Functional MRI (fMRI) may detect abnormalities within the brain that cannot be found with other imaging techniques. fMRI can detect or measure changes in metabolism, blood flow, regional chemical composition, and absorption.
Functional imaging research finds that those with Alzheimer’s typically have reduced brain cell activity in certain regions. For example, studies with fluorodeoxyglucose (FDG)-PET scans indicate that Alzheimer’s is often associated with reduced use of glucose in brain areas important in memory, learning, and problem-solving. An FDG-PET scan is considered a reasonable test for those with a recent diagnosis of dementia and documented cognitive decline of at least six months who meet diagnostic criteria for both Alzheimer’s and frontotemporal dementia (Alzheimer’s Association, 2024j).
MOLECULAR NEUROIMAGING
Molecular imaging uses specialized tracers to detect specific molecular changes associated with the disease at a cellular level.
Molecular imaging modalities include positron emission tomography (PET) and single-photon emission computed tomography (SPECT), which provide detailed pictures of what is happening inside the body at the molecular and cellular level and involve the use of radiopharmaceutical agents that bind to or are taken up by a target issue of interest. Amyloid PET imaging represents a potential major advance in the assessment of those with cognitive impairment. Amyloid PET scanning makes amyloid plaques “light up” on a brain PET scan, enabling, for the first time, accurate detection of plaques in living people, which in the past could only be detected by examining the brain at autopsy (UCSF, 2024e; Alzheimer’s Association, 2024j).
Functional Assessment
Dementia is characterized by cognitive deficits that cause functional impairment to basic and instrumental activities of daily living. Functional status can be assessed by use of a validated tool, direct examination of the patient, or obtaining information from a knowledgeable informant (i.e., family member or friend who routinely observes the person in his or her day-to-day activities). An assessment of the patient’s functional status should include, at minimum, an evaluation of the ability to perform instrumental activities of daily living (IADLs) (i.e., preparing meals, managing finances, etc.) and basic activities of daily living (ADLs) (i.e., eating, dressing, etc.).
As Alzheimer’s progresses, periodic assessment of the patient’s ability to function should be carried out. Functional status can also be assessed using one of a number of valid and reliable instruments. Examples include:
- Lawton Instrumental Activities of Daily Living Scale
- Barthel ADL Index
- Katz Index of Independence in Activities of Daily Living
- Functional Activities Questionnaire (FAQ)
- Functional Independence Measure (FIM)
- Performance Assessment of Self-care Skills (PASS)
(Physiopedia, 2024a)
FUNCTIONAL ACTIVITIES QUESTIONNAIRE (FAQ)
The FAQ is efficient to administer, taking 10 minutes or less to complete. It evaluates activities of daily living and is completed by an informant who spends at least two days a week with the person and rates the person in the following 10 areas:
- Writing checks and maintaining other financial records
- Assembling tax or business records
- Shopping alone for clothes, household necessities, or groceries
- Playing a game of skill or working on a hobby
- Heating water for coffee or tea, turning off the stove
- Preparing a balanced meal
- Keeping track of current events
- Paying attention to and understanding a TV show, book, or magazine
- Remembering appointments, family occasions, holidays, or medications
- Traveling out of the neighborhood (e.g., driving or arranging to take the bus)
FAQ rating:
- 3 points if dependent on others to complete the activity
- 2 points if requires assistance to complete the activity
- 1 point if has difficulty with the activity, but performs independently
- 1 point if never performed the activity and would have difficulty now
- 0 points if performs the activity independently with no difficulty
- 0 points if never performed the activity but could do so now
Scoring:
- Scores range from 0 to 30, with higher scores indicating more functional difficulty.
- Scores higher than 10 suggest reduced functional ability.
(Statistics Solutions, 2024)
PHARMACOLOGIC AND MEDICAL MANAGEMENT
At this time, there are no medications that can cure Alzheimer’s. The U.S. Food and Drug Administration, however, has approved treatments that address the underlying biology of the disease. Other medications may help lessen symptoms such as memory loss and confusion. These medications fall into two categories:
- Drugs that change disease progression in those living with early AD
- Drugs that may temporarily mitigate some symptoms
Anti-Amyloid Treatments
Lecanemab (Leqembi) is an anti-amyloid antibody intravenous infusion therapy administered every two weeks. It is approved to treat early Alzheimer’s disease in those who have confirmation of elevated beta-amyloid in the brain. This is the second therapy to demonstrate that removal of beta-amyloid reduces cognitive and functional decline in those with early Alzheimer’s.
Donanemab (Kisunla) is an anti-amyloid antibody intravenous infusion therapy administered every 4 weeks. It is approved for those who have mild cognitive impairment or mild dementia due to Alzheimer’s disease and who have confirmation of elevated beta-amyloid in the brain. This is the third therapy that demonstrates that the removal of beta-amyloid from the brain reduces cognitive and functional decline (Alzheimer’s Association, 2024k).
ADUCANUMAB (ADUHELM) DISCONTINUED
Aducanumab (Aduhelm) is an anti-amyloid antibody intravenous infusion therapy administered every 4 weeks. It received accelerated approval from the FDA to treat early Alzheimer’s, including in patients who are living with mild cognitive impairment or mild dementia due to Alzheimer’s disease who have confirmation of elevated beta-amyloid in the brain. This was the first therapy to show that removing beta-amyloid from the brain reduces cognitive and functional decline in people with early Alzheimer’s disease.
It was approved despite lack of clear clinical evidence demonstrating cognitive benefits. Initially, it was priced at $56,000 per year, which was later reduced to $28,200. Aducanumab, however, was discontinued on November 1, 2024, so the manufacturer can “reallocate resources to other Alzheimer’s disease treatments” (Brockman et al., 2023; Biogen, 2024).
Treating Cognitive and Memory-Related Symptoms
As Alzheimer’s progresses, brain cells are damaged and cognitive symptoms worsen. While the following medications do not stop the damage caused by AD, they may help temporarily lessen or stabilize symptoms related to memory and thinking by affecting chemicals involved in carrying messages among and between the brain’s nerve cells (Alzheimer’s Association, 2024k).
CHOLINESTERASE INHIBITORS
Cholinesterase inhibitors are prescribed to treat symptoms related to memory, thinking, language, judgment, and other thought processes. These drugs support communication between nerve cells by preventing the breakdown of acetylcholine, a chemical messenger important for memory and learning. These medications include:
- Donepezil (Aricept), approved for all stages of Alzheimer’s
- Rivastigmine (Exelon), approved for mild to moderate Alzheimer’s and Parkinson’s diseases
- Galantamine (Razadyne), approved for mild to moderate Alzheimer’s
Side effects include nausea, vomiting, loss of appetite, and increased frequency of bowel movements (Alzheimer’s Association, 2024k).
GLUTAMATE
Cholinesterase inhibitor and glutamate regulator combination of donepezil and memantine (Namzaric) is approved for moderate-to-severe Alzheimer’s disease. Side effects may include nausea, vomiting, loss of appetite, increased frequency of bowel movements, headache, constipation, confusion, and dizziness.
ANTIOXIDANT VITAMIN E
Vitamin E (alpha-tocopherol) has been studied in the treatment of Alzheimer’s. Overall, the data suggest that vitamin E at a dose of 2,000 international units per day confers a modest benefit in delaying functional progression in patients with mild to moderate Alzheimer’s, with no measurable effect on cognitive performance (Press & Buss, 2024).
DRUGS WITH UNPROVEN BENEFIT
Several other therapies have been studied in patients with dementia, with largely negative results, including:
- Estrogen replacement
- Anti-inflammatory drugs
- Ginkgo biloba
- Vitamin B supplement
- Omega-3 fatty acids
(Press & Buss, 2024)
Drugs for Management of Noncognitive Symptoms (Behavioral and Psychological)
Behavioral and neuropsychiatric symptoms are common, and, as mentioned earlier, are often more problematic than memory impairment. Such symptoms can include:
- Depression
- Anxiety
- Apathy
- Agitation
- Aggression
- Aberrant motor disturbance
- Aberrant vocalizations
- Hallucinations
- Delusions
- Disinhibition
- Sleep disturbances
- Wandering
Antipsychotic drugs may be prescribed for people with dementia who develop changes such as aggression and psychosis, but usually only after other drugs have been tried. Many atypical antipsychotics are used “off label” to treat dementia-related behaviors, but brexpiprazole (Rexulti) is the only atypical antipsychotic with FDA approval to treat agitation associated with dementia due to Alzheimer’s. It is not approved for the treatment of dementia-related psychosis without agitation.
The FDA requires that all atypical antipsychotics carry a safety warning that the medication has been associated with an increased risk of death in older patients with dementia-related psychosis.
Other antipsychotics may be prescribed for hallucinations, delusions, aggression, agitation, hostility, and uncooperativeness, including:
- Aripiprazole (Abilify)
- Clozapine (Clozaril)
- Haloperidol (Haldol)
- Olanzapine (Zyprexa)
- Quetiapine (Seroquel)
- Risperidone (Risperdal)
- Ziprasidone (Geodon)
(Alzheimer’s Association, 2024l; Press, 2024)
Antidepressants may be used “off label” in patients with dementia. Selective serotonin reuptake inhibitors (SSRIs), in particular citalopram (Celexa), are useful in the management of agitation and paranoia, since symptoms are often driven by a mood disorder that is poorly verbalized. Trazodone is an alternative and is often used for sleep onset.
Anxiolytics may be prescribed for anxiety, restlessness, verbally disruptive behavior, and resistance, including:
- Lorazepam (Ativan)
- Oxazepam (Serax)
Analgesics may be prescribed for pain, which is an important source of behavioral issues. Using analgesics requires careful monitoring to balance risks and benefits of pain treatment versus persistent pain. Adequate pain control may be observed as improvements in behavior and function. Nonopioids are preferred over opioids for treating mild persistent pain. Recommended medications include:
- Acetaminophen
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Antidepressants for chronic neuropathic pain:
- Tricyclic antidepressants (TCAs)
- Selective noradrenalin reuptake inhibitors (SNRIs)
- Selective serotonin reuptake inhibitors (SSRIs)
Opioid analgesics are the mainstay for treating moderate to severe pain in patients with advanced illness. Long-acting or sustained-release analgesic preparations should be used for continuous pain. Breakthrough pain should be identified and treatment with fast-onset, short-acting preparations and by avoiding meperidine and methadone. These analgesics may include:
- Morphine
- Oxycodone or hydrocodone with or without acetaminophen
- Hydromorphone
- Tramadol
- Fentanyl
- Buprenorphine
Anticonvulsants may be effective for neuropathic pain and include:
- Gabapentin
- Pregabalin
- Carbamazepine
(OPG, 2024)
Managing Coexisting Health Problems
Comorbid health issues, alone or in combination, can further diminish the patient’s ability to function. For example, people who do not see or hear well may be easily confused in unfamiliar situations. Couple those limitations with Alzheimer’s disease, and the confusion intensifies. Recognition and treatment of any and all coexisting conditions can help improve the patient’s functional ability and quality of life.
IMPAIRED VISION AND HEARING
For a person with dementia, sight and hearing loss can cause extra problems, such as confusion about what’s happening around them and problems with communication.
There are many causes of sight loss in people with dementia, including:
- Eye conditions, such as cataracts or macular degeneration
- Other health conditions, such as stroke
- Normal aging of the eye
However, people with dementia can also have visual difficulties due to dementia’s effects on parts of the brain that handle visual information. This means they will have visual problems even with healthy eyes.
People living with dementia and sight loss may experience more disorientation, greater problems with mobility, and increased risk of falls. They will also have more difficulties with communication, understanding and learning new tasks, completing activities, and increased social isolation.
Medicare beneficiaries diagnosed with dementia are less likely to receive eye care than those without diagnosed dementia. Routine eye care services such as regular eye exams are excluded from Medicare coverage. However, Medicare does cover certain eye care services if a person has a chronic eye condition, such as cataracts or glaucoma.
It is often difficult to separate the signs of hearing loss from those of dementia, and often one condition may mask the other. If the person has hearing loss, it can make diagnosing dementia more difficult. They may have difficulties with answering some of the questions asked in an assessment, or their hearing loss may mask the difficulties they are having.
Medicare does not pay for hearing aids, including examinations for prescribing or fitting hearing aids, though in some cases implants to treat severe hearing loss are covered (Alzheimer’s Society, 2024i; Medicare Interactive, 2024).
DEPRESSION
Depression is a common problem for people with dementia, which is often diagnosed when a person is in the early stage of the condition, but can develop at any time. Some people may become depressed as a reaction to negative events or not having positive experiences to look forward to. Others may become depressed as a result of damage to part of the brain involved in mood. Dementia increases the risk of suicide, particularly during the first few months after a dementia diagnosis has been made.
The symptoms of dementia, such as problems with memory and thinking, can make it harder to treat depression.
In the earlier stage of dementia, many find that talking with a counselor or therapist can be helpful, as well as attending support groups. Antidepressant medication is widely used to treat depression. However, it doesn’t seem to be as effective in people with dementia. Depression may be helped more by improving a person’s quality of life through:
- Providing care and support that matches their needs, personality, and preferences
- Addressing any underlying issues that may be contributing to depression, such as loneliness or pain
- Ensuring they have opportunities to do things that provide pleasure or fulfilment
(Alzheimer’s Society, 2024j)
REHABILITATION FOR PERSONS WITH DEMENTIA
The goals of rehabilitation for persons with dementia are to help maintain or improve higher cognitive function and engagement in daily activities to the extent possible as the disease progresses, devise strategies to compensate for declining function, and provide caregivers with the education and skills they need to create a supportive environment and reduce disability.
Occupational therapy, physical therapy, and speech-language pathology services can be of great benefit to patients with dementia as well as to their family members and other caregivers. (See also “Supportive Care for the Person with Alzheimer’s Disease” below.)
Occupational Therapy (OT)
Occupational therapists typically focus on five areas of human occupation when working with patients with dementia. These areas include:
- Activities of daily living (i.e., eating, hygiene, dressing, mobility, toileting)
- Instrumental activities of daily living (i.e., care of others, household management, safety, home maintenance, transportation)
- Rest and sleep
- Leisure
- Social participation
The goals of the occupational therapist are to maximize the patient’s involvement in both ADLs and IADLs, promote safety, and enhance a patient’s quality of life. OT practitioners focus on identifying the patient’s remaining abilities rather than on their deficits and look for ways to maintain and prolong the person’s independence.
EVALUATION PROCESS
Occupational therapists evaluate patients with dementia to determine their strengths, impairments, and performance areas that need intervention and to help patients retain existing function for as long as possible. When working with dementia patients, occupational therapists use a family-centered model that includes family caregivers in all aspects of the process.
The process begins with an occupational profile, an analysis of occupation, and the use of standardized and nonstandardized assessment tools to evaluate specific domains, such as those described in the table below. During the evaluation process, the occupational therapist also identifies caregiver concerns about occupational performance and the handling of difficult behaviors.
| Domain | Tool |
|---|---|
| (AbilityLab, 2024; AOTA, 2024; Coyne, 2021; Encyclopedia of Mental Disorders, 2024; MD+CALC, 2024; Physiopedia, 2024a, 2024b, 2024c; University of Illinois, 2024; University of Pittsburgh, 2024; Wiley, 2023) | |
| Activities of daily living |
|
| Instrumental activities of daily living |
|
| Leisure |
|
| Motor skills |
|
| Cognitive function skills |
|
| Physical environment |
|
| Nutrition |
|
| Accompanying conditions |
|
OCCUPATIONAL THERAPY INTERVENTIONS
As part of the interdisciplinary team, occupational therapists provide evidence-based interventions throughout the continuum of care and across the entire health spectrum for Alzheimer’s patients and families, setting up a program to meet the goals of safety, independence, utilization of retained abilities, and improved quality of life for the patient as well as family and caregivers.
During Early Stages
In the early stages of Alzheimer’s, patients are generally able to function well in daily life. Interventions focus on compensating for loss of cognitive abilities and recognizing remaining abilities. Patients may start to notice they are forgetting simple things like appointments, where they placed their keys, or if they’ve taken their medications. They may need reminders to bathe or eat. At this point, memory aids such as calendars, journals, medical reminders, and daily routine schedules can help maintain independence with high-level ADLs. When these aids are combined with caregiver education, further improvement in patient independent outcomes occur and caregiver stress is reduced.
During Middle Stages
During the middle stages of the illness there is more decline in memory and high-level cognition. Most individuals begin to require assistance with basic self-care tasks such as going to the bathroom in time. They may experience decreased sequencing ability and motor planning of basic ADLs.
During this stage, the person should be allowed to continue their routine, and caregivers are instructed to avoid jumping in and doing everything for the person. With cues and prompting, the person can still physically assist with their routine and is encouraged to do so in order to avoid loss of that automatic skill.
During ADL retraining with dementia patients, increased verbal or visual cues, demonstration, physical guidance, partial physical assistance, and problem solving can improve outcomes.
During Later Stages
In the final stage of the disease process, individuals are likely no longer be oriented to person, place, or time. They are often dependent in all or most self-care activities, including feeding. They may experience severe loss of motor control and may need to use a wheelchair as a result.
Due to this decreased ability to physically perform self-care tasks, the focus is on educating caregivers on safe transfers, contracture management through home exercise programs, proper positioning to avoid skin breakdown and increase comfort, and providing enjoyable sensory stimulation.
Caregivers at this stage may also be experiencing greater stress, depression, and exhaustion. Referral to online or in-person support groups may be of help (Stromsdorfer, 2022).
INTERVENTION TECHNIQUES
Listed below are several evidence-based techniques occupational therapists use when working with persons with dementia.
Positive Approach to Care (PAC)
PAC is an educational program that helps family caregivers and professionals better understand the changes in the brain and how it feels to be living with dementia. PAC provides practical advice on ways to connect and interact with people who have dementia and focuses on doing things with rather than to the person. PAC encourages and prepares caregivers to:
- Respond rather than react to a person with dementia changes and abilities in a way that is proactive
- Appreciate that, with practice, common reactions to the person with dementia can become thoughtful responses that improve everyone’s quality of life
- Recognize that the person with dementia is doing the best they can when faced with challenging situations
- Change their approach, behavior, and expectations for improved outcomes
- Modify the physical and sensory environment (lighting, sound, activity) to promote function and satisfaction
(Alzheimer Society of Canada, 2024)
PAC includes the following elements:
- Positive Physical Approach (PPA): a 6- to 9-step method to approach and connect with a person, resulting in a reduced risk of agitation, surprise, and negative reactions; greater mutual understanding and comfort; and easier task completions
- Positive Personal Connection (PPC): five phrases that help care partners connect with the person before jumping into a task, resulting in more positive outcomes
- Positive Action Starters (PAS): five phrases that help care partners receive less resistance and refusals when getting started with a care task
- Hand under Hand (HuH): a technique to support and assist a person living with dementia with ADLs by utilizing remaining muscle memory to help the brain process the situations
(Teepasnow.com, 2024)
Skills2Care
Skills2Care is an innovative and evidence-based occupational therapy program consisting of training and certification designed to target functional limitations and environmental barriers with the goal of having a direct community impact by helping to increase independent living for older adults with physical and cognitive changes, as well as their families (University of Pittsburg, 2024).
Validation Therapy
Validation therapy is a method of therapeutic communication that can be used to connect with someone who has moderate to late-stage dementia. It places more emphasis on the emotional aspect of an interaction and less on the factual content, thereby imparting respect to the person, their feelings, and their beliefs. In essence, the caregiver enters the person’s world in order to reduce their anxiety. The idea behind this form of therapy is that people in the late stages of life may have unresolved issues that drive their behaviors and emotions. The way caregivers or family members respond to these behaviors and emotions can either make them worse or help resolve them. Validation therapy acknowledges the individual even if what they’re saying isn’t factual (Shuman & Altman, 2024; Heerema, 2024).
Reality Orientation
Reality orientation is an approach in which the environment, including dates, locations, and current surroundings, is frequently pointed out and woven into conversations with the person. This typically involves:
- Talking about orientation, including the day, time of day, date, and season
- Using people’s names frequently
- Discussing current events
- Referring to clocks and calendars
- Placing signs and labels on doors, cupboards, and other objects
- Asking questions about photos or other memorabilia
Reality orientation techniques have been shown to slow cognitive decline and may even improve cognitive functioning. In addition, the use of reality orientation may help to delay nursing home placement (Heerema, 2022b).
Reminiscence
This approach uses all the senses to help a person with dementia recall and talk about their life story. It has been shown to improve mood, well-being, and some mental abilities such as memory. It involves talking about things from the past using prompts such as photos, familiar objects, or music (Saragih et al., 2022).
Tailored Activity Program (TAP)
TAP is an occupational therapy intervention that involves assessments of cognitive and functional abilities and interests of the person living with dementia, caregiver readiness and availability to use activities, and the physical environment for supports of daily function and engagement in activities. OTs then design activities tailored to assessment profiles and instruct caregivers in their daily use, along with providing disease education and stress reduction techniques (Pizzi et al., 2023).
Environmental-Based Methods
Environmental-based methods pertain to the arrangement of a space and the objects within it as well as sensory elements to address a dementia patient’s behaviors and perceptions. These may include, but are not limited to:
- Making necessary changes to promote safety, comfort, and independence, such as installing handrails, removing trip hazards, and ensuring adequate lighting
- Employing design considerations such as contrasting colors, clear signage, and familiar decor to reduce confusion and promote a sense of familiarity
- Allowing personalization of living space with familiar items and photographs to promote a sense of identify and a feeling of being at home
- Providing sensory stimulation with appropriate lighting, colors, and textures to reduce agitation and promote relation
- Simplifying the layout and design of the home
- Minimizing clutter
- Regulating noise
(Dave, 2023)
CASE
Louise, an 82-year-old woman with Alzheimer’s disease, has been placed in the nursing facility where Emelia works as a certified nursing assistant. The staff are just beginning to get to know Louise and learning what approaches work best when caring for her.
Emelia has found that Louise insists things are different than what they are. For example, she claims she has to go and get the mail or make dinner for her kids and becomes very agitated and resistant to any attempts at redirection. This often results in caregiver frustration.
Emelia had recently attended the training session provided by Parker, the facility’s occupational therapist, in which various techniques for working with dementia patients were presented. Emelia remembered the validation method, and when Louise became agitated and insistent that she could not leave her room to go to breakfast because she had to feed her chickens, Emelia entered her world by asking her to talk about her chickens. As Louise talked to Emelia and answered her questions, Emelia guided her out of her room, down the hallway, and into the dining room for breakfast.
Physical Therapy (PT)
Physical therapy plays a vital role in enhancing the quality of life, managing symptoms, and promoting overall health for persons with Alzheimer’s. In the early and middle stages of AD, physical therapists assist people to stay as mobile as possible. This helps them to maintain a degree of independence and to continue to perform their roles in the family and in the community.
Later, as AD progresses, physical therapists assist in maintaining an individual’s ability to perform daily activities for as long as possible, thereby reducing the load on family members and caregivers and possibly delaying the need for facility-based care. In the end stages of disease progression, physical therapy intervention may shift to more palliative measures such as positioning, bed mobility, maintaining range of motion, and recommendation of and fitting for appropriate assistive devices/equipment.
PHYSICAL THERAPY INTERVENTIONS
- Mobility enhancement: Physical therapists design exercises and mobility programs tailored to the individual’s needs that work to strengthen muscles, enhance balance, and reduce risk of falls, with the ultimate goal of promoting greater independence.
- Functional training: Physical therapists conduct functional training sessions that mimic the activities needed to perform activities of daily living. This many include walking, rising from a chair, or climbing stairs. Functional training helps individuals with AD maintain their abilities for as long as possible, thereby enhancing quality of life and reducing dependency.
- Pain management: Physical therapists use a combination of techniques, including manual therapy and therapeutic exercises, to manage pain and alleviate muscle tension. This enhances comfort and also fosters relaxation and overall well-being.
- Gait training: Physical therapists analyze gait patterns, identify any abnormalities, and work to correct them, including recommending and training in the safe use of mobility assistive devices (e.g., canes, walkers, wheelchairs). Proper gait training helps reduce the risk of falls and promotes mobility and self-confidence.
- Balance improvement: Balance issues are common among Alzheimer’s patients, making them more susceptible to falls and injuries. Physical therapists employ specialized balance exercises to improve balance and stability. These exercises are tailored to each patient’s needs and capabilities, ensuring that the exercises are safe and effective.
- Swallowing and feeding support: As the disease progresses, patients may face challenges related to swallowing and feeding. Physical therapists provide guidance on proper body positioning and techniques that make swallowing and feeding safer and more efficient.
- Cognitive and memory enhancement: Physical activities can indirectly impact cognitive function; physical therapists guide the patient in physical activities and exercises that may enhance cognitive abilities, promote mental well-being, and provide a sense of accomplishment.
- Quality of life improvement: Physical therapy aims to improve the overall quality of life for Alzheimer’s patients. Addressing mobility, pain, and discomfort enables individuals to maintain a degree of independence and engage in activities they enjoy as much as possible.
- Individualized care: Physical therapists provide individualized care plans, tailoring their approach to the specific challenges and goals of each patient.
- Family and caregiver education: Physical therapists often work closely with family members and caregivers, providing them with education on exercises and techniques that can be incorporated into daily routines to support the patient’s well-being.
(Breakthru Physical Therapy, 2024; Physiopedia, 2024b)
ASSESSMENT TOOLS
Timed Up and Go (TUG) Test is used to determine fall risk and to measure the progress of balance, sit-to-stand, and walking. It is designed for people with impairments including Alzheimer’s disease. This test requires a chair with an armrest, a stopwatch, and a tape measure to mark off 3 meters (approximately 10 feet). The patient is seated in the chair. The stopwatch is started following the therapist’s command to the patient to stand up and walk the measured distance, turn around, walk back to the chair, and sit down. The stopwatch is stopped when the patient is seated. Time to complete the task is averaged over two trials; if a patient takes 14 seconds or longer, they are classified as high risk for falling.
Tandem Stance Test assesses the individual’s balance. The patient is asked to place one foot directly in front of the other, touching heel to toe. A chair can be used as needed to attain this position. Holding this position tests lateral postural stability by narrowing the base of support. The length of time the person is asked to hold this position is commonly 10–30 seconds.
Portable gait testing mat is a gait analysis system that analyzes a patient’s ambulatory biomechanics. It measures gait for both time (temporal) and space (spatial) through pressure sensors in the mat. The patient is asked to walk on the mat walkway, and software converts the sensor data into foot placement patterns and overall gait patterns. The mat provides valid and reliable walking measurements such as footfall patterns, step length, cadence, and speed, and can measure changes in walking or gait patterns through replication of real-life scenarios.
Global Deterioration Scale (GDS)/Reisberg Scale is a commonly used scale that divides cognitive decline into seven stages to better understand how well a person thinks (cognitive decline) and functions (physical abilities). This test is most relevant for persons with Alzheimer’s disease since some other forms of dementia, such as frontotemporal dementia, do not always include memory loss.
Pain Assessment in Advanced Dementia (PAINAD) is a reliable assessment tool used with patients who have advanced dementia and are judged potentially to be in pain. The scale requires close and attentive observation of the patient’s breathing, vocalizations, facial expressions, and body language. Each is graded from 0 to 2, with 0 being normal, 1 being abnormal, and 2 being extremely abnormal. A score of 1–3 is interpreted as mild pain, 4–6 as moderate pain, and 7–10 as severe pain.
Pain Assessment Checklist for Seniors with Limited Ability to Communication (PACSLAC) is a more intensive observational pain tool with five subscales: facial expression, activity and body movement, social personality, mood, and “other” (such as changes in eating and sleeping behaviors).
Functional Assessment Staging Tool (FAST) describes seven progressive stages of Alzheimer’s disease. It is a cognitive staging scale that can assist in identifying lost and preserved cognitive function. Findings are used to better identify interventions to enhance quality of life and reduce care burden and the costs associated with progressive cognitive impairment. The tool is recommended for use on initial examination and whenever assessing changes in cognitive function and dysfunction. FAST is determined through interview or report from an informant and/or by observation of patient performance.
(Dementia Care Central, 2023a; Iowa GeriatricPain.Org, 2024).
DEVELOPING A PLAN OF CARE
Physical therapy for patients with dementia focuses on optimizing and/or preserving balance, muscle strength, and functional mobility; preventing falls; and providing pain management and maximized safety in the home or facility setting. Physical therapists consider both patient and caregiver needs when developing a treatment plan, which can include behavioral, cognitive, mental, physical, and functional domains.
The plan of care should enhance retention of the patient’s remaining capabilities and appeal to the patient’s individual abilities and interests. Incorporating familiar objects or actions into the physical therapy regimen for patients with Alzheimer’s disease and other dementias may prove helpful. Finding out what motivates the individual patient and incorporating favorite pastimes into the therapy plan allows for emotional development and increased feelings of comfort.
Therapeutic exercises may need to be tailored to accommodate cognitive limitations. Intense, multimodal programs (which may include passive, active, or resistive exercises, as well as gait, balance, and/or endurance training) may become overwhelming for some patients, such as:
- Those with limited attention spans
- Those who are easily overstimulated by verbal instructions
- Those who become anxious/agitated when presented with excessive transitions between tasks
Physical therapists must often use nonlanguage interactions based on awareness of a patient’s tolerance for interpersonal engagement, cognitive fatigue, or sensory overload. Communication can be a major issue when working with patients with dementia, particularly in an instructional, task-oriented setting such as physical rehabilitation. Various communication strategies and teaching techniques for patients with dementia that may be helpful include:
- Verbal cueing: using short, simple, or one-step verbal instructions
- Visual cueing: pointing to an object or gesturing a movement
- Tactile cueing: taking a patient’s hand to indicate going for a walk
- Mirroring: serving as a “mirror” by demonstrating a desired movement to the patient
- Task breakdown: breaking down tasks into short, simple steps to be completed separately
- Chaining: after mastering the steps in a task, linking them together into one fluid movement
- Active-assisted facilitation: taking the patient’s hand or other body part and helping to move it through a desired motion
- Muscle memory training: training a person’s procedural memory via motor repetition, in order to help the body more automatically respond to changes such as uneven or unstable surfaces
Physical therapists may involve the patient’s family and caregivers in the treatment plan, instructing in strategies for maintaining routines and using cues to initiate motor tasks. Family education may include ergonomic training to help family members/caregivers more safely perform tasks such as how to safely assist patients with bed mobility, transferring, and ambulation, as well as how to correctly use and maintain adaptive equipment or assistive devices. Ergonomic education and home safety assessments may help minimize risk of injury to both patients and caregivers.
In later stages of the disease, when cognitive decline is more pronounced, physical therapy interventions may shift to focus on more palliative measures, such as:
- Slowing the rate of functional/motor decline
- Instructing caregivers in proper joint positioning/mobilization to minimize risk of contractures
- Instructing caregivers in appropriate patient positioning/mobilization to minimize risk of pressure injuries or skin tears
- Instructing caregivers in the use and maintenance of higher-level assistive equipment, such as mechanical lifts, wheelchairs, etc.
Home assessments and safety recommendations can help make the home environment safer and may help delay the need for facility-based care (Staples, 2021; Sponholz, 2021).
CASE
Mr. Hartman, a 68-year-old retired professor, was diagnosed with Alzheimer’s disease approximately one year ago and is referred to physical therapy for evaluation and treatment of increased falling in the home and community. During his initial evaluation, Mr. Hartman seems ill at ease and shows inconsistent ability to follow directions. Mr. Hartman’s wife tells the physical therapist that she is concerned about her husband’s safety when walking in their yard, as he has fallen twice there, but that he loves to watch the birds come to their neighbor’s backyard feeder.
Upon completing the evaluation, the therapist determines that Mr. Hartman demonstrates significant decreases in lower extremity strength, static and dynamic standing balance, and safety awareness. Having learned from his wife that Mr. Hartman enjoyed working as a carpenter’s assistant during summer vacations when he was growing up, the therapist obtains a simple kit to assemble and paint a wooden bird feeder and centers his physical therapy sessions around this familiar activity.
While it is difficult for Mr. Hartman to follow complex instructions related to specific repetitive exercises, he is easily able to pedal a seated lower extremity ergometer while sanding the bird feeder pieces; practice repeated sit <–> stand transfers while assembling the feeder; and work on balance by retrieving paints, sandpaper, and pictures of birds from different parts of the therapy gym (on high shelves, off the floor, etc.) with close supervision. By the third physical therapy session, Mr. Hartman appears more at ease, and his wife states that he even seemed eager to come and work on his project today.
Speech-Language Pathology (SLP)
SLP plays a central role in screening, assessment, diagnosis, and treatment of persons with dementia. Speech and language therapy for dementia may involve assessment, therapy programs, support groups, advice, and education. SLPs may use standardized and nonstandardized assessment tools and a variety of other data sources, including clinical observations in the home or long-term care setting.
An SLP manages dysphagia in persons with dementia using the following interventions:
- Environmental modifications (e.g., increasing lighting, limiting distractions, use of naturalistic settings, adaptive seating for postural adjustments, verbal and/or visual prompts or cues)
- Diet modifications (e.g., altering food texture, temperature, or taste) to facilitate increased intake or improved swallowing ease and safety
- Compensatory strategies for feeding and swallowing (e.g., alternating bits of food with slips of liquid), including supervision and/or cognitive support to ensure consistent use of these strategies
- Additional strategies for maximizing independence and safety (e.g., shopping lists, weekly meal planners, and written cues to sequence mealtime activities or to identify steps for using safe-swallow strategies)
SLPs perform hearing screening of individuals with cognitive decline and make referrals to audiologists for full screening and appropriate management (ASHA, 2024).
SUPPORTIVE CARE FOR THE PERSON WITH ALZHEIMER’S DISEASE
Supportive care is an interdisciplinary effort focusing on preventing and relieving suffering and on supporting the best quality of life for patients and their families facing serious illness. Effective supportive care for patients with advanced dementia can improve the patient’s symptoms, lessen the burden on caregivers, and help to ensure that treatment decisions are well informed and reflect the patient’s and family’s values.
Addressing Challenges to Care
Caring for a person with Alzheimer’s disease can be challenging, no matter the setting—hospital, institution, or the home.
CARE CHALLENGES IN ACUTE CARE SETTINGS
People with dementia have a high risk of being admitted to an acute care hospital, which are institutions with strong traditions and rigid structures that concentrate on medical interventions and less on psychosocial care. This may conflict with the needs of those with dementia, as psychosocial interventions are especially important to this population. In addition, staffing levels, skill mix, and skill deficits can impede adequate care for those with dementia in a hospital setting.
Hospitalization for a person with dementia is associated with increased risk for functional and cognitive decline, falls, delirium, and increased morbidity and mortality. Patients with dementia may see the acute care environment as unstable, chaotic, and unsafe, which may result in heightened confusion, fear, disorientation, anxiety, agitation, and aggressive behavior. This makes nursing more challenging by putting nurses at risk of physical and verbal abuse and by impacting the nurses’ ability to optimize patient care.
Nurses caring for dementia patients in acute care settings can experience frustration and negative feelings resulting from inadequate resources, opportunities, or abilities to perform quality care for patients with dementia. In addition, a lack of knowledge of the complex needs of these patients causes frequent emotional exhaustion and stress for nurses. Nurses who work with a deficit in knowledge and skills might feel a sense of professional failure and frustration while providing care for patients with dementia.
In acute care, nurses must prioritize the needs of those patients who have acute illnesses and are not routinely trained in caring for the dementia patient or in effective strategies that can be applied in managing their behavioral issues (Karrer et al., 2022; Çevik et al., 2022; Boltz et al., 2023).
Responsive Behaviors
People with dementia require structure and order in their environment, a definite challenge in a fast-paced acute care setting. Nursing staff must approach the dementia patient with an easygoing, unrushed attitude or else experience negative responsive behaviors, defined as words, movements, or actions that dementia patients use in an effort to make their needs known. Examples include:
- Grabbing on to others
- Wandering
- Yelling and screaming
- Biting
- Pushing
- Throwing things
- Cursing
- Hitting
- Kicking
- Restlessness
- Repetitive sentences or questions
- Making noises
- Sexually inappropriate behaviors
Experiencing these responsive behaviors in the workplace causes feelings of anger, fear, and sadness and is perceived by healthcare professionals as one of the difficult aspects of providing care for dementia patients in acute care settings. Often, as a result, the patient is prescribed sedating medications, which can result in adverse reactions.
The following are strategies that have been found to be effective when providing care for a person with dementia.
- Monitor personal comfort: Check for pain, hunger, thirst, constipation, full bladder, fatigue, infections, and skin irritation.
- Avoid being confrontational or arguing about facts: If a person says they want to go visit a parent who died years ago, don’t tell the person that the parent is dead. Instead, say, “Your mother must be such a nice person. Tell me about her.”
- Redirect that person’s attention.
- Remain flexible, patient, and supportive by responding to the emotion, not the behavior.
- Create a calm environment by avoiding noise, glare, and too much background distraction.
- Allow adequate rest between stimulating events.
- Acknowledge requests and respond to them when feasible.
- Look for reasons behind each behavior and rule out any causes related to medications or illness.
- Don’t take the behavior personally; share experiences with coworkers.
(Alzheimer’s Society, 2024k)
Involving Family and Caregivers
Nurses welcome the familiarity that caregivers provide the patient in the hospital. Such familiarity helps reduce responsive behaviors. Family and other caregivers understand what is normal for the patient, and this helps the nursing staff identify changes that may be indicative of delirium, pain, or other treatable conditions and provide more patient-centered care.
Caregivers of people with dementia are often dissatisfied with the quality of care in hospitals. They are concerned about nurses’ recognition and understanding of dementia, the social interactions of the nursing staff with the patients, the patients’ and caregivers’ involvement in decision-making, and aspects of dignity and respect. Caregivers often feel that they are the only ones who represent the patient’s interests.
When a person with dementia is admitted to an acute care hospital, good communication, involvement with, and cooperation between nurses and caregivers is essential. It is important to keep open lines of communication with family caregivers. Besides utilizing the care recommendations they may provide, the nursing staff can also let caregivers know what is going well. Small successes are shared with the family, which can help promote the patient’s successful transition into and out of the acute care setting (Keuning-Plantinga et al., 2021).
PROVIDING PATIENT-CENTERED CARE IN ALL SETTINGS
A diagnosis of Alzheimer’s presents a number of challenges to the planning of care. Patient participation should be encouraged, but expectations should be aligned to the person’s abilities. Goals should be planned without expectation of dramatic improvement.
Whether the person is cared for at home or another care setting, overall treatment goals are the same: to maximize the person’s functional abilities and quality of life and to provide competent, compassionate care that acknowledges and respects the person and family. Ideally, that care is multidisciplinary, including medicine, nursing, social work, occupational therapy, physical therapy, and speech-language pathology.
The challenges of caring for someone with Alzheimer’s disease include communicating effectively with the person; assisting with ADLs while helping maintain the person’s independence; planning activities that will help maintain well-being and prevent boredom; and managing behavior problems such as agitation, wandering, and sleep disturbance. Meeting these challenges may become more difficult as the disease progresses.
Providing a Safe Home Environment
As dementia progresses, physical and social environments prove ever more difficult for the person, and a safe environment is essential. Things to consider in creating such an environment include:
- Adding strong, low-glare lighting, and night lights; arranging lights to prevent or minimize shadows; and using motion-activated lighting in hallways and bathrooms
- Covering electric outlets in every room
- Lightening the color of walls to reflect light and contrast with the floor and avoiding busy patterns
- Marking the edges of stairs with brightly colored tape for better visualization
- Limiting the size and number of mirrors, which may confuse a person with AD
- Placing decals at eye level on sliding glass doors and picture windows
- In the bathroom:
- Installing a freestanding or built-in shower chair or a transfer seat for the bathtub
- Installing grab bars
- Installing a handheld shower
- Adding a raised toilet seat
- Installing temperature-controlled and motion-activated water faucets
- Removing the lock from the door
- Securing medicine and other items in a lockbox
- Blocking access to cleaning supplies and razors
- Using large nonskid bath mats
- Setting the water heater to 120 ˚F to avoid scalding tap water
- In the bedroom:
- Installing an audio (“baby”) monitor
- Removing the lock from the door
- Installing bed rails or a hospital bed to facilitate getting in and out of bed
- Placing a bell or a horn at bedside to summon help
- Using motion-activated night lights
- In hallways and stairs:
- Installing railings on both sides of a staircase to help with stability
- Ensuring carpets on stairs are not loose
- Using sturdy, nonslip pads or double-sided tape to affix rugs to the floor
- In the kitchen:
- Disconnecting the garbage disposal
- Locking some cabinets with childproof locks
- Unplugging the microwave
- Regularly checking the pantry and refrigerator for spoiled food
- Installing temperature-controlled and motion-activated water faucets
- Installing childproof knob covers and devices that turn off a cooktop, oven, or range after a set time to protect against burns or fires
- Placing certain regularly eaten food items within reach and stashing other foods out of sight
- Removing pet food after feeding
- Discarding toxic plants and decorative fruits to prevent mistaking for real food
- Removing vitamins, prescription drugs, sugar substitutes, and seasonings from the kitchen table and counters and placing in a locked drawer or cabinet
- In the living room and home office:
- Anchoring bookcases to the wall
- Clearing surfaces
- Considering adaptive furniture such as motorized lifts
- Installing combination or key locks on rooms and other storages places containing potentially dangerous/hazardous items
- Keeping car keys out of reach
- Storing firearms in a gun safe or off the property
- Listing emergency phone numbers (ambulance, poison control, doctors) near all phones
(Van Dyke & Dono, 2023; Alzheimer’s Association, 2024l)
Creating a Supportive Environment
A supportive environment includes both physical and social aspects that work together to support the unique needs and abilities of the person with dementia. A supportive and dementia-friendly environment helps people reach their full potential and does not cause needless dependency. This results in an improved quality of life for people with dementia, their family members, and caregivers.
An environment can help support or hinder social connection and sense of self. It can give independence or force dependency. Independence is about what matters to a person: how they feel is just as important as physical independence. Dementia-friendly environments empower people.
A homelike supportive environment provides continuity and familiarity in everyday life, encourages family involvement, and strengthens social ties. The following elements are included in designing and creating a homelike environment:
- Providing unobtrusive clinical support
- Individualizing care
- Practicing flexible problem-solving for individual care issues
- Using language common to the home, not to healthcare
- Supporting continuation of roles and lifestyles
- Designing daily life around stimulating interests and pastimes that give pleasure, add variety, and make use of skills and abilities
- Offering freedom of movement, choice of activity, and individual control and decision-making wherever possible
- Providing safety and security while supporting independence
- Encouraging meaningful relationships
- Encouraging engagement in daily-life experiences
- Designing environmental cues to highlight the purpose of different spaces and location of items
- Creating smaller-scale, homelike living spaces:
- Welcoming dining areas
- Homelike furniture and furnishings
- Personalization of bedrooms
- Warm colors
- Spaces and rooms for small groups to promote a sense of extended family
- Continuous indoor and outdoor spaces
- Being sensitive to culture, religion, and spirituality
- Inviting family participation
- Adopting a flexible management and supervisory approach
(Victoria Department of Health, 2022)
Some basic principles for creating and maintaining such an environment both in the home and in a healthcare facility include:
- Make change very slowly. Carefully prepare the person for any change in medications, nutrition, therapy, personnel, or location. Consistency in staff assignment has a calming effect on the person. The person should stay in the same room with the same roommate whenever possible.
- Keep the person active as long as possible. Daily exercise, outdoors if possible, helps maintain physical and emotional function. Activities should be focused on making the person more comfortable and designed to allow them to use existing skills to perform familiar tasks. Avoid complexity in activities, as this can create anxiety.
- Maintain a routine. A person with dementia generally feels more secure when routines are established and followed closely.
- Provide social stimulation without overload and encourage and maintain communication through every possible channel. Keep communications short and simple.
- Give a choice of activity and involvement. Provide different options for both indoor and outdoor activities in which the person takes either an active role or watches others.
- Avoid crowds and large spaces without boundaries. Try to prevent sensory overload and provide boundaries and interior landmarks that are easily visible. This can be done using contrasting colors to demarcate boundaries.
- Keep noise low or masked by the sound of music that was popular during the person’s youth.
- Play older television shows to provide a familiar background and anchor the person in a period they can remember.
- Monitor nutrition, attention to mouth and teeth, and footwear. Help with eating and oral hygiene can reduce the risk of infection. Comfortable, well-fitting shoes with nonslip soles help prevent falls.
- Provide positive input. Praise and compliments for any achievement help maintain the person’s self-esteem and encourage self-participation in activities of daily living.
- Provide reality checkpoints, such as calendars with large days and dates, clocks with large numbers marking the hours, and reminders of special events such as birthdays, anniversaries, and holidays. Signage with figures illustrating the use of an area is helpful in orientation. The presence of personal items can help the person identify an unfamiliar room as their own.
- Support bowel and bladder control. A consistent toileting routine helps preserve function and control. Use clothing with simple fasteners like Velcro (hook and loop) or pants with elastic waistbands.
- Assist with activities of daily living. The person frequently will have problems attending to basic hygiene and daily life activities. Drinking, eating, bathing, or dressing may require careful attention in order to avoid infections and eventual progression to a generally debilitated state.
- Attend to the person’s appearance. Keep the person clean and free from odors, dress the person in their own clothes, and keep them well-groomed. This aspect of care is noticed by family members.
- Closely attend to emerging symptoms and identify a person’s problems before they become unmanageable. Keep in mind that the person is often unable to describe routine physical symptoms, even pain.
- Support family caregivers. Commend their efforts, refer them to support groups, and assist them in creating a helping network. Families caring for a loved one at home may require referrals to agencies offering respite care. Assist them in ways to understand and respond to a person’s behavior and communication.
- Offer information and referrals for legal matters, advance directives, end-of-life care, etc. This should be done at the time of diagnosis, while the person with Alzheimer’s can still have a voice in the decisions made.
Communication Issues
As Alzheimer’s dementia progresses, the person’s ability to communicate begins to deteriorate, and the following changes occur:
- Difficulty finding the right words
- Not understanding what words mean
- Repetitious use of familiar words
- Describing familiar objects rather than calling them by name
- Inventing new words for familiar objects
- Decreasing attention during longer conversations
- Losing one’s train of thought
- Reverting back to native language
- Problems with organization of words
- Reduction in efforts to speak
- Relying on gestures more than speaking
Communicating with the person who has Alzheimer’s disease begins with patience, respect, understanding, and remembering that the person is not deliberately being difficult. If the individual has a vision or hearing deficit, it is always important to make certain hearing aids and glasses are being worn so as to avoid additional barriers to communication.
General strategies for communicating with someone who has dementia include:
- Making eye contact and calling the person by name
- Being aware of one’s tone, volume, facial expressions, and body language
- Encouraging a two-way conversation if the person is able
- Being open to the person’s concerns, even if they are hard to understand or address
- Being patient with angry outbursts
- Allowing more time for the person to respond and not interrupting
- Not talking about the person as if they’re not there
- Not using “baby talk” with the person
- Using nonverbal methods, such as a gentle touch to guide the person or holding their hand when speaking to them
(NIA, 2024b)
Strategies to communicate more effectively with someone in the early stage of Alzheimer’s include:
- Not making assumptions about the person’s ability to communicate
- Not excluding the person from conversations
- Speaking directly to the person rather than to their caregiver or companion
- Being patient and taking time to listen to the person express their thoughts, feelings, and needs
- Giving the person time to respond and not interrupting unless the person requests help
- Asking the person what help may be needed
- Discussing which method of communication is most comfortable, which may include face-to-face conversation, email, or phone calls
- Using humor to lighten a mood and make communication easier
- Not pulling away and remembering that honesty, friendship, and support are important
In the middle stage of Alzheimer’s, as the disease progresses, the person will have more and more difficulty communicating. Helpful strategies may include:
- Engaging with the person in one-on-one conversation in a quiet place with minimal noise and other distractions
- Speaking slowly and clearly, keeping sentences simple, and focusing on one idea at a time
- Facing the person and maintaining eye contact
- Giving the person adequate time to respond
- Being patient and offering reassurance to encourage expression of thoughts
- Asking one question at a time
- Asking yes-or-no questions; avoiding open-ended questions
- Avoiding correcting or criticizing; listening and attempting to find the meaning in what is being said
- Repeating what the person has said for clarification
- Making statements rather than asking questions (e.g., instead of asking if the person needs to go to the bathroom, saying, “The bathroom is here”)
- Avoiding criticizing or correcting
- Avoiding arguing
- Offering clear, step-by-step instructions for tasks
- Giving visual cues or demonstrating tasks to encourage participation
- Attempting written notes when spoken words seem confusing
In the late stage of Alzheimer’s, the person may rely on nonverbal communication such as facial expressions or vocal sounds. The following communication techniques are helpful during this stage of the illness:
- Approaching the person from the front and identifying oneself
- Encouraging nonverbal communication; to understand what the person is saying, asking them to point or gesture
- Using touch, sights, sounds, smells, and tastes as a form of communication
- Considering the feelings behind words or sounds; emotions are often more important than what is being verbally expressed
- Avoiding talking down to the person and do not talk to others about the person as if they aren’t there, since even those unable to speak may still be able to hear and understand
- Using positive body language, such as relaxing, leaning forward, and smiling
- Repeating the message as often as necessary
- Distracting the anxious or agitated person
(Alzheimer’s Association, 2024m)
Ethical lying, also known as therapeutic lying, is an essential skill to develop. Because dementia patients must be kept safe and avoid becoming agitated and in distress, less-than-truthful communication may be required in order to accomplish these goals. It can, in some cases, be kinder to ethically lie than to be completely honest.
For example, reminding the person that a loved one has died can make the person upset and sad. Ethical lying can also help to ease difficult situations, such as when the patient needs to stop driving due to safety concerns. Having the car “disappear” or suggesting it has been stolen can be a more compassionate way to manage what is often a devastating loss for the patient. This also allows the caregiver to be perceived as being on the side of the patient and not the one who is taking away the right to drive (Piedmont Healthcare, 2024).
Nonverbal communication, especially touch, between caregivers and those with Alzheimer’s is also important. As dementia progresses, nonverbal communication may become the main way a person communicates. Nonverbal communication may also become necessary when non-native English speakers have reverted to their native language. Strategies include:
- Using physical contact to communicate interest and to provide reassurance
- Not sitting too close to the person or standing over them to communicate, as it can feel intimidating; instead, sitting or standing at eye level
- Avoiding sudden movements, a stern tone of voice, or tense facial expressions, which can upset or distress the person, even if the words being said are not upsetting
- Matching body language and facial expression to what is being said, even if this might feel a bit forced at times
- Learning to recognize what a person is communicating through their body language; trying to keep them engaged if they seem distracted or bored
- Using visual prompts such as cue cards or a book of pictures a person can point at to communicate wants and needs
- Allowing the person to use drawing or singing to express themselves
Persons with Alzheimer’s may ask the same question repeatedly because they do not remember the answer given. The caregiver can respond to the question and then try to distract the person with an activity or a change of topic or a change of scene (Alzheimer’s Society, 2024l).
Supporting Basic Activities of Daily Living (ADLs)
To persons with Alzheimer’s disease, the tasks of daily living can be frustrating and overwhelming. During early Alzheimer’s disease, a person will begin to demonstrate a lack of attention to personal hygiene and grooming. They soon forget to bathe, change clothes, or use the bathroom. It is important to remember that support for ADL function must recognize the person’s functional ability and extent of cognitive impairment and provide person-centered care practices.
Activities of daily living are actually quite complicated when broken down into steps. Brushing one’s teeth, for example, requires recognition of the equipment used (toothbrush, toothpaste, sink) and remembering how to use each piece of equipment. In addition, the person must remember to find the equipment, put the toothpaste on the toothbrush, brush the teeth, and rinse the mouth.
- Activity analysis (task breakdown) is useful in assisting persons with Alzheimer’s disease to function on their own. This entails determining the manual and cognitive activities involved in the completion of a task and organizing the task into manageable sections.
- Verbal coaxing allows the person to perform the activity, and when they complete the task, the ability will be retained longer.
- Providing cues such as labeling, placing equipment and clothes out in view, and offering demonstrations are all useful.
- Establishing and maintaining a routine in ADLs helps the person retain learned skills longer, and therefore need less assistance. Once the routine becomes automatic, the person no longer needs to stop and think what to do next. A fixed routine for eating and toileting also reduces the incidence of incontinence.
- Offering choices can be helpful. With patients who refuse to attend to daily activities, such as bathing, it can be more effective to ask them “when” they would like to bathe instead of “if” they want to bathe.
BATHING AND ORAL CARE
It is common for persons with dementia to refuse to bathe. Bathing can be a challenge because persons with Alzheimer’s disease may be frightened by showers or they may resist the invasion of privacy that comes with bathing in front of a caregiver. They also may believe they’ve already showered or bathed and that it is not reasonable to do so again. Creative means of managing resistance include:
- Avoiding a discussion as to whether a bath is needed or not
- Waiting and trying again later if the person adamantly refuses to bathe
- Providing partial baths when daily bathing or showering is not needed
- Following the person’s previous routines, including time of day and type of bathing preferred
- Preparing the bathroom in advance
- Always checking the temperature of the water
- Completing one step at a time, talking through each step
- Being calm and gentle; not rushing and hurrying
- Using a seat and handheld shower attachment, which can be less frightening for the person
- Introducing warm shower water gradually, starting at the feet and moving up the body
Many people with dementia do not like to have their hair washed at all; there are dry shampoo products available that can be used when this is an issue. An electric razor can be used for shaving to reduce the risk of cuts, especially if the person is taking blood-thinning medications such as warfarin.
Oral hygiene is often avoided by persons with dementia, who may resist doing it and not allow anyone else to do it for them. One solution is to provide an oral care swab (e.g., Toothette) with diluted hydrogen peroxide solution. If the person consistently refuses to cooperate with oral care, fresh fruit such as apples can also aid in cleaning the teeth (Piedmont Healthcare, 2024; Alzheimer’s Association, 2024n).
DRESSING AND GROOMING
Self-esteem for a person with dementia is still important, and past grooming habits should be considered, as well as the person’s style and cultural clothing preferences. Dressing assistance may include:
- Providing simple garments with large zipper pulls, Velcro fasteners, and few buttons; simplifying dressing with sweatsuits and pull-on pants and shirts
- Using cardigan sweaters instead of pullovers
- Laying out clothes in the order in which they will be put on
- If needed, providing constant repetition of each step
- Using nonskid shoes, such as washable rubber-sole shoes with Velcro fasteners, or slip-ons
- Continuing the person’s regular grooming routines and favorite beauty shop or barber
- Maintaining hairstyles, beards, and makeup the way the person liked to appear before developing dementia
- Assisting with combing hair, trimming fingernails, shaving, etc., since the person may forget the purpose of items like combs, nail clippers, razors, etc.
- Performing tasks along with the person and encouraging the person to copy one’s motions
Inappropriate dressing may be one of the problems faced by caregivers. The person may no longer be able to coordinate colors, may put a shirt on backward, or may fasten buttons in the wrong order. Often persons will put on many layers of clothes or may want to remove clothing at inappropriate times.
If the person wants to wear the same clothes every day, duplicates can be made available while the other set is laundered. Many older persons, with or without Alzheimer’s, feel embarrassed when completely undressed, so removing and replacing one article of clothing at a time may work better (Piedmont Healthcare, 2024; Alzheimer’s Association, 2024n).
TOILETING
Caregivers must understand that the person with Alzheimer’s may no longer respond to signals such as the urge to void or defecate, may forget where the bathroom is located even if they’ve been in the same house for years, and may forget what to do when they do find the bathroom. Family members and other caregivers may feel awkward when assisting with toileting, although this usually subsides over time. A matter-of-fact, calm, and reassuring manner with the person with dementia is the best approach.
Communication challenges often increase in the late stage of the disease. It may be necessary to encourage the person to use the toilet and to watch for cues, such as:
- Restlessness or fidgeting
- Making unusual sounds or faces
- Pacing
- Pulling at clothes
- Sudden silence or hiding in corners
The person may also use trigger words or phrases to indicate the need to use the toilet, and these words may be unrelated to toileting.
Other strategies to assist with toileting include:
- Checking the location of mirrors in the bathroom, since people with dementia may confuse their reflection for someone else already in the room and not go in because they think the toilet is occupied
- Removing other objects in the environment that can be mistaken for a toilet, such as large planters, since the person may not recognize the toilet as the appropriate place to void
- Providing a bedside commode or urinal if getting to the bathroom is a problem, especially at night
- Posting a colorful sign on the bathroom door to help the person identify the room
- Setting a regular schedule for using the bathroom
- Monitoring mealtimes and foods consumed so as to predict when it’s time to use the bathroom
- Respecting the person’s privacy as much as possible
- Assisting with removing or adjusting clothing as necessary
- Helping the person get in the right position, if needed
- Giving cues if the person doesn’t know what to do; talking through each step
- Setting a urinary alarm system for reminders
When assisting the person to a public restroom, go together—don’t let the person enter the restroom alone. If a female caregiver is assisting a male dementia patient, use the women’s room and, if necessary, hand out cards to startled strangers stating the person has dementia. The same is true for a man caring for a woman. Many places now have all-gender bathrooms that may be utilized.
When the person suddenly loses bowel or bladder control, a medical evaluation should be done to rule out medical conditions such as urinary tract infection or medication side effects. When the person becomes incontinent, the following suggestions are helpful:
- Restricting fluid and caffeine intake 2–3 hours before bedtime
- Using incontinence aids such as disposable briefs and pads for beds and chairs or condom catheters for men at night
- Dressing the person in manageable clothing and considering eliminating underwear
Constipation and fecal impaction can also cause a great deal of discomfort and lead to unwanted behavioral problems. It is extremely important for caregivers to continually assess and monitor the person’s bowel function. Avoid using laxatives, but encourage a high-fiber diet (Piedmont Healthcare, 2024; Alzheimer’s Association, 2024n).
EATING
Eating habits and behaviors change during the course of Alzheimer’s disease, which may be caused by physiologic or psychological factors. Proper nutrition reduces the risk of constipation, dehydration, and vitamin deficiency. These conditions contribute to increased confusion and a decline in physical functioning.
In early-stage Alzheimer’s, depression related to the diagnosis may result in anorexia and weight loss. Persons may forget to eat or refuse to eat. Confusion and agitation may lead to extreme eating behaviors such as gorging. It is also important to monitor what the person eats and drinks, as some inappropriate items may be eaten, such as soap or other household items. All items that look like food, such as dog biscuits, flower bulbs, artificial fruits, etc., should be secured so they are not eaten.
In the later stage of the illness, profound memory loss interferes with the recognition of food, the need to eat, and the mechanics of eating. In addition, the person may become resistant to being fed. A nutritionist can make suggestions during this stage, and evaluation by a speech pathologist is indicated when the person begins to have trouble swallowing.
Physiologic factors affecting eating behaviors may include dental problems such as uncomfortable dentures, missing teeth, and/or periodontal (gum) disease. Neurofibrillary tangles and plaques can affect the function of the hypothalamus, which regulates appetite, hunger, and thirst signals. Many persons with Alzheimer’s lose their sense of smell, which affects taste and appetite. Some medications can also affect appetite.
In addition to depression, other psychological factors that affect eating behaviors include new and unfamiliar environments, which create confusion and agitation; distractions such as loud noises; unappealing food; and unusual odors such as urine. Such factors are quite variable, since individuals progress through the stages of the illness at their own pace and in their own physiologic manner.
Supporting the Eating Process
The following are helpful ways to assist the person with dementia to eat and to enjoy the process of eating:
- Providing a quiet, relaxing, and homelike atmosphere
- Ensuring the dining area is well lit
- Maintaining familiar dining routines
- Reducing distracting stimuli
- Playing soothing music during meals to decrease agitation
- Facilitating social eating with others in the earlier stages of the disease; limiting social stimulation in the later stages
- Allowing the person to choose mealtimes or adjust times based on agitation or disorientation
- Offering food choices, but limiting the number
- Putting only one utensil and one food in front of the person at a time
- Offering culturally appropriate foods
- Keeping the table free of clutter
- Using white dishes to help distinguish food from the plate, and using placemats of contrasting color to help distinguish the plate from the table (since patterned plates, bowls, and linens may be confusing)
- Providing bendable straws or lidded cups for liquids
- Checking the food temperature
- To prevent overeating, limiting access to food between meals, maintaining a schedule, and monitoring intake
- Providing ample time to eat; not rushing the person
- Ignoring messy eating (since it is more important for the person to eat than to be tidy)
- Sitting to the side and level with the person, making eye contact, and speaking with the person while assisting with eating
- Modeling the sequence of eating, and reminding the person to eat slowly and chew thoroughly
- When necessary, reminding the person to swallow the food
- Providing verbal prompts or physical cues if required to encourage the person to eat
- Encouraging independence when possible
- Adapting foods (e.g., finger foods) and providing assistance when utensils can no longer be used
- Providing functionally appropriate foods and beverages to match swallowing capability
- Using adaptive devices/utensils as needed
- Using hand-over-hand feeding when appropriate
(Piedmont Healthcare, 2024; Alzheimer’s Association, 2024n)
CASE
Mr. Florio often came to the nursing home on Sundays to take his wife out to lunch at a local restaurant. He observed that sometimes his wife would eat everything on her plate, but at other times she would not eat anything at all. A search was begun for an explanation, and Mr. Florio noted that when his wife faced the wall of the restaurant, she cleaned her plate, and when she faced the staff, other customers, or the cash register, she failed to eat at all. It became clear that the distractions offered by the busy restaurant produced her failure to eat.
Maintaining Nutritional Well-Being
Weight loss is common among persons with Alzheimer’s, regardless of quality of care. Wandering, restlessness, and agitation expend energy and interfere with food intake. In mid- and late-stage Alzheimer’s disease, persons are unable to feed themselves or to chew and swallow the food when it is placed in their mouths.
The following steps can support the nutritional well-being of persons with dementia:
- Provide a balanced diet with a variety of foods, including vegetables, fruits, whole grains, low-fat dairy products, and lean protein foods.
- Limit foods with high saturated fat and cholesterol; limit fats such as butter, shortening, lard, and fatty cuts of meats.
- Offer nutrient-dense foods.
- Cut down on refined sugars, which contain calories but lack vitamins, minerals, and fiber. But in the later stages of Alzheimer’s, if loss of appetite is a problem, adding sugar to foods may encourage eating.
- Limit foods with high sodium and use less salt; use spices or herbs to season foods as an alternative.
- As the disease progresses, loss of appetite and weight loss may require supplements between meals.
- To maintain hydration, encourage fluids throughout the day or foods with high water content such as fruit, soups, milkshakes, and smoothies.
- Lack of physical exercise will decrease appetite. Encourage simple exercises such as going for a walk.
Monitoring the person’s nutritional status for weight loss and possible nutritional deficiencies also includes:
- Reviewing medications to check for drugs that may affect appetite
- Assessing for vision problems that may cause confusion at mealtime
- Assessing for depression
Ensuring Proper Swallowing
Those who are unable to swallow properly can become dehydrated and aspirate food, leading to aspiration pneumonia.
- Assess the person’s ability to swallow food. Remind them to swallow with each bite and show them how. Gently stroke the throat to promote swallowing, and at the end of the meal, check the person’s mouth to make sure food has been swallowed.
- Prepare foods so they are not hard to chew or swallow. Grind foods, cut food into bite-size pieces, or serve soft foods such as cottage cheese, scrambled eggs, mashed potatoes, or applesauce. Avoid foods such as popcorn or raw vegetables.
- Thicker liquids such as fruit nectars, milkshakes, and eggnogs are easier to swallow and less likely to cause choking. It may be necessary to use thickening agents in liquids in order to avoid aspiration.
A speech-language pathologist can assess the person’s needs and make recommendations (Alzheimer’s Association, 2024n; Piedmont Healthcare, 2024).
AMBULATING
Alzheimer’s and other types of dementia can affect areas of the brain that are responsible for motor control, coordination, and balance. Decreased mobility becomes more prominent as Alzheimer’s progresses, increasing the risk for becoming disabled or leading an entirely inactive lifestyle. Persons in the later stages may also gradually lose the ability to stand or get themselves up from a chair. They are also more likely to fall.
As physical activity lessens due to cognitive decline and loss of independence, muscle weakness can become a significant issue. This lack of strength can further exacerbate mobility issues such as difficulty standing up from a chair or climbing stairs.
Alzheimer’s also can lead to spatial disorientation, making it challenging for persons to navigate their surroundings and perform tasks that require movement and coordination, such as walking to a different room or stepping around obstacles. Signs of balance problems include:
- Unsteady gait
- Shuffling rather than fully lifting feet off the ground
- Shortened steps
- Bumping into things
- Stooped posture
- Turning using multiple small steps, rather than by pivoting
Balance is considered a skill, and as such, it can be improved. Almost any physical activity that safely gets a person moving is good for Alzheimer’s symptoms, but low-impact workouts that include light resistance activities may be particularly helpful in improving balance, in part by strengthening leg muscles and maintaining bone density.
Physical therapy can play a crucial role in managing mobility issues. Therapists design individualized programs that help maintain muscle strength, improve balance, and reduce the risk of falls.
Recent studies have shown the benefits of music-cued exercises for people with dementia. A twice-weekly, home-based physical therapy program of music-cued gait training may help some people with mild to moderate AD increase their ambulation speed.
Some other ways to help the person move around more easily can include:
- Making the home safer by continually picking up and putting away any obstacles on the floor that the person could trip over or need to steer around
- Keeping useful items within reach so the person does not need to strain to reach or use a stepping stool
- Keeping the house well lit, especially at night
- Providing nonskid slippers and shoes
Canes and walkers may help maintain balance, but there are important issues to consider. Canes and walkers may actually increase the likelihood of falls—especially if used incorrectly—due to the increased complexity of using a device while walking, which requires the ability to multitask. It is important for the patient to receive appropriate training in the use of any new assistive devices, preferably by a physical or occupational therapist, and for caregivers to consistently monitor usage for any difficulty or decline in the patient’s ability to use such devices, with or without assistance.
Novel surfaces may affect gait speed in those with very mild Alzheimer’s, such as walking on shiny surfaces that appear icy or slick. Waiting in a line, taking a few steps forward, and stopping can become confusing. Getting in and out of cars can take longer.
As the disease progresses, many individuals can gradually lose the ability to walk. In later stages they may require actual physical support by a caregiver to walk. As the disease progresses, the ability to ambulate even with assistance may be lost. Eventually the person may need a wheelchair in order to feel and be safe.
In the later stages of severe dementia, the person frequently loses the ability to sit up without assistance, requiring some form of external physical support such an arm rest, lap belt, or other external device to keep from sliding down in the chair.
Some people with dementia eventually become largely confined to a bed or chair. When this point is reached, a health professional should be consulted about specialized equipment options, as well as to provide caregiver education in how to safely move the patient without causing injury to either patient or caregiver (Dementia Care Central, 2023a; Alzheimer’s New Zealand, 2024).
Supporting Instrumental Activities of Daily Living (IADLs)
Performing IADLs is the first ability to decline in persons with Alzheimer’s disease, while the ability to perform basic activities initially remains unimpaired. Instrumental activities of daily living that healthcare professionals may be asked to assess and to assist with include shopping and meal preparation, driving and other transportation needs, managing medications, and physical and social activities.
SHOPPING AND MEAL PREPARATION
In the early stages of Alzheimer’s, the person may begin to lose skills needed to shop for and prepare proper meals. Caregivers can assist the person to complete a menu and a shopping list. The list should be organized so that the items are divided up based on their location in the store to make it easier for the person to find things when shopping. Once persons with dementia become confused about their surroundings and cannot count properly, it is unwise to let them go shopping alone. At this point caregivers can accompany the person to the store.
It is important to keep the person involved in preparing food and drinks to help maintain skills and interest in eating and drinking. It is often helpful to break down meal preparation into individual tasks.
Other suggested activities for a person with dementia who is living alone or who needs extra support with meals include:
- Buying frozen, refrigerated, or ready-to-eat meals, which typically require little preparation and may help the person prepare food more easily
- Having meals delivered, such as through the Meals on Wheels program
- Shopping online if the person has difficulties going to the store
- Using simple notes about where certain foods are stored or placing pictures on cupboards or the refrigerator to assist the person in locating items
- Providing simple written instructions to help the person prepare, cook, or reheat food
- Planning meals that do not require any cooking, such as salads or sandwiches
(Alzheimer’s Society, 2024n)
Cooking can be very unsafe once coordination becomes impaired, as the person may let cooking gas escape, leave a burner on, and/or allow loose clothing to catch fire. If the person is continuing to cook, do not leave the person alone in the kitchen. Over time, caregivers should eventually take over cooking. Persons may get up at odd times and try to cook. If they are no longer able to cook safely, keep the kitchen locked (Dementia Care Notes, 2024).
DRIVING AND TRANSPORTION NEEDS
Driving is a complex task requiring an ability to make and execute split-second decisions. Dementia affects the ability to process many different pieces of information at the same time. Because of this, a person living with Alzheimer’s will, at some point, be unable to drive safely.
Once the diagnosis of Alzheimer’s disease is established, healthcare professionals encourage the family to discuss the issue of driving with the person. Each state has its own laws and policies regarding physician reporting of driving with dementia to the Department of Motor Vehicles. The person with dementia may be required to report to the DMV for a behind-the-wheel driver reexamination. In some states those diagnosed with moderate or severe dementia may have their licenses automatically revoked. Healthcare professionals should be aware of the regulations in their own state and local jurisdiction.
In the early stages of Alzheimer’s disease and other dementias, some people may still possess the skills necessary for safe driving. Most dementia is progressive, however, and symptoms such as memory loss, visual-spatial disorientation, and decreased cognitive function worsen over time and eventually require that the person give up driving. Warning signs of loss of ability to drive safely include:
- Getting lost in familiar areas
- Not observing traffic signs or signals
- Being confused about traffic signs
- Making slow or poor decisions in traffic
- Driving either too slowly or too fast
- Getting angry or confused while driving
- Responding in a delayed fashion
- Swerving into wrong lanes
- Stopping in the middle of traffic
- Confusing the accelerator and brake pedals
- Hitting curbs
- Incorrect signaling
- Riding the brake
- Judging distances poorly
- Receiving traffic tickets or warnings
- Being involved in car crashes
Research has suggested that people living with Alzheimer’s disease overestimate their driving abilities and that caregivers can more accurately identify unsafe driving. It may be difficult to determine at what point an individual can no longer drive safely.
An occupational therapist with special expertise in driving rehabilitation may be recommended to perform a driving assessment. However, even if someone with early dementia passes a driving assessment, continued driving is very closely monitored as the dementia progresses.
It can be very upsetting for the person to lose the independence that driving provides, and this may pose a dilemma for caregivers. However, it is generally accepted that those who refuse to quit driving even though they pose a hazard must be prevented from doing so. It is usually best to completely remove the person’s car once they are diagnosed with dementia. If it remains at the home, the vehicle should be permanently disabled and all car keys taken away.
When driving is no longer an option, it is important to make alternative transportation arrangements so that the person’s mobility and activity level are not unduly restricted. Groceries, meals, and prescriptions can be delivered to the home. Rides can be provided by:
- Family
- Friends
- Neighbors
- Public transportation
- Taxis
- Senior and special needs transportation services
(Piedmont Healthcare, 2024; FCA, 2024a)
MANAGING MEDICATIONS
Healthcare professionals working with patients with Alzheimer’s disease have an important role in helping family caregivers take on the task of medication management. Surveys have shown that family caregivers of Alzheimer’s patients may feel ill prepared and unsupported by healthcare professionals. Healthcare professionals can be effective in easing their concerns, making recommendations by carefully reviewing all medications, providing guidance on how to simplify the medication regimen, and making recommendations for problems such as patient resistance in taking medications. Consulting with a pharmacist can also be helpful.
The following information can be taught to family caregivers regarding how to manage patient medications:
- Make taking medications a normal part of the daily routine by pairing it with specific events throughout the day, such as mealtimes or before going to bed.
- Use a pill box organizer and utilize a daily log of what medications are to be taken and when.
- Use simple language and clear instructions.
- If the person refuses to take the medication, stop and try again later.
- If swallowing is a problem, ask if the medication is available in another form or if the medication can be crushed and mixed with food.
- Keep medications stored in a locked drawer or cabinet and not left out where the person can find them.
- Know that herbal therapies and over-the-counter medications can interact with prescribed medications.
- Understand how to determine if medications are effective. For example, the patient may not be able to articulate pain but may be calmer and more easily engaged after receiving pain medication.
- Know which medications are priorities as well as which medications can safely be skipped now and then when the patient is resistant to taking them.
- Give medications with meals, if allowed, and administer the most important medications first.
- Alternatively, with the prescriber’s approval, give medications in the morning, when agitation is less likely to occur.
- Make certain the patient is wearing glasses and hearing aids, if needed, to minimize confusion.
- If a person refuses to take the medication, stop and try again later.
- To cope with resistance, give medications covertly in food or drink. Covert administration can prevent exacerbation of a coexisting medical problem that could lead to the need for hospitalization (e.g., a patient with heart failure requiring diuretics).
- Create a list of distraction activities (e.g., listening to a favorite piece of music) to employ when the patient is resistant so that taking medication is more pleasant.
- Do not argue or try to convince the patient to take medications, since this can increase tension and agitation.
- Should resistance become routine, talk about medication options with the healthcare provider to see if some medications can be discontinued or given in an alternate form.
- Should the patient begin to have difficulty swallowing pills, request assistance from a healthcare provider or pharmacist to determine if the medication is available in another form. Do not crush a pill or tablet without first discussing with a physician or pharmacist.
- Acknowledge that mistakes will happen and develop a plan for dealing with errors that does not place emphasis on blaming.
- Know the types of medication mistakes that can happen, such as giving the wrong dose, and when and how to notify the healthcare provider if this occurs.
- Have emergency numbers easily accessible. If a medication overdose is suspected, call poison control or 911 before taking any action.
(Alzheimer’s Association, 2024o)
Healthcare providers should provide caregivers with a copy of the written care plan. It should reinforce the teaching points described above and include the phone numbers for prescribing healthcare professionals who can provide assistance. Reassess medication management goals of care every six months and document them clearly.
PROVIDING PHYSICAL AND SOCIAL ACTIVITIES
Persons with Alzheimer’s disease and other dementias may withdraw from activities, family, and friends. It is very important, however, to maintain these connections, as they reduce the effects of memory impairment and lead to a better quality of life. Social and cognitive stimulation can help maintain general well-being and prevent boredom and agitation in people with Alzheimer’s disease, especially in the early stages of the disease. Such stimulation can also encourage self-expression, lessen anxiety and irritability, make the person feel more engaged, and stir memories.
Walking provides good exercise and may relieve tension and stress. It may also help increase appetite. Simple exercises that encourage increased range of motion can help maintain optimal muscle functioning.
Keep things simple, as the confused person has a short attention span and may become easily frustrated when faced with multiple-step tasks or activities. Gardening, painting with watercolors or finger paints, drawing, and coloring are good ways for confused people to express themselves.
Research shows that activities done in the past can still resonate with the older person who has advancing dementia. For example, a retired business executive may enjoy sitting at a desk with files filled with papers, and someone who spent years as a homemaker may enjoy domestic activities like folding laundry or sweeping.
Keep activities on an adult level, though children’s toys can be usefully adapted in the later stages of dementia, e.g., some people often respond positively to baby dolls. The person may enjoy playing familiar games such as cards, bingo, or board games, or doing simple jigsaw puzzles.
Musical activities are usually successful whether the individual is making the music themself or just listening. Music, including group sing-alongs, can help recall pleasurable moments from the past. Dance music from their youth might encourage the person to dance.
Plan social visits for times when the person feels best and in environments that are calm and quiet. Busy settings full of noise and people are often stressful for the person with dementia.
Books, newspapers, magazine articles, and family photo albums can serve as cues for reminiscence and provide an opportunity for family conversation in which the person may still participate.
Certain television programs can be of great interest. Animal programs are often much enjoyed by those with dementia. It is important to know that some TV shows can be upsetting, particularly violent, suspenseful, or horror shows, since the person may no longer be able to understand what is real and what is fiction.
Other activities may include:
- Dance
- Tai chi
- Yoga
- Swimming
- Joining a walking group
- Arts-based activities (e.g., drawing/painting classes, drama groups)
Many people with dementia regularly attend adult day programs specifically for those with dementia. It is common for the person to resist initially, but eventually they often enjoy the new routine and social activities (Piedmont Healthcare, 2024; NHS, 2024).
Watching for Elder Abuse in Dementia Patients
People with Alzheimer’s disease or other cognitive impairment are at higher risk of abuse than other older adults. Up to 5 million older Americans are abused every year, yet one study estimated that only 1 in 24 cases of abuse are reported to authorities. Types of abuse include:
- Physical: causing physical pain or injury
- Emotional: verbal assaults, threats of abuse, harassment, and intimidation
- Neglect: failure to provide necessities, including food, clothing, shelter, medical care, or safe environment
- Abandonment: an extreme version of neglect, in which a caregiver who has assumed responsibility for the person deserts or abandons them, most often in their own home or after a hospital stay, but also in public locations or with law enforcement
- Confinement: restraining or isolating the person other than for medical reasons
- Financial: misuse or withholding of the person’s financial resources by another (which is estimated to cause $28.3 billion in losses each year)
- Sexual: touching, fondling, intercourse, or any sexual activity when the person is unable to understand, unwilling to consent, threatened, or physically coerced
- Willful deprivation: denying a person medication, medical care, shelter, food, therapeutic device, or other physical assistance, and exposing that person to the risk of physical, mental, or emotional harm, except when the older, competent adult has expressed a desire to go without such care
(NCEA, 2024; NCOA, 2024a)
Abusers are both women and men and people of all ages. An analysis of calls to the National Center on Elder Abuse resource line found that family members were the perpetrators in nearly 47% of incidents; medical (nonfamily) caregivers were the perpetrators in almost 13% of causes; and only 6.7% of callers did not know their abuser (NCOA, 2024b).
RECOGNIZING ELDER ABUSE
Although currently there is no gold standard for screening people with dementia for signs of abuse, healthcare providers are in a position to identify abuse.
Signs of physical abuse can include:
- Unexplained bruises, cuts, or sores
- Pressure marks
- Broken bones, sprains, or serious injury, especially without a reported fall
- Abrasions
- Burns
Signs of emotional abuse can include:
- Unexplained withdrawal from normal activities
- Isolation from family or friends
- Sudden change in alertness
- Depression
- Frequent arguments between caregiver and the person
- Increased fear or anxiety
- Unusual changes in behavior or sleep
Signs of financial abuse may include:
- Fraudulent signature on financial documents
- Unpaid bills
- Unusual or sudden changes in spending patterns, will, or other financial documents
Signs of neglect can include:
- Pressure injuries
- Unattended medical needs
- Poor hygiene
- Unusual weight loss
- Unsanitary living conditions
Signs of sexual abuse can include:
- Bruises around the breasts or genital area
(NCOA, 2024b)
CAREGIVER STRESS AND ELDER ABUSE
One of the leading causes of elder abuse is caregiver stress and other problems that prevent caregivers from properly caring for older adults. Factors such as substance abuse or financial problems can lead to caregiver abuse of elders in both residential and institutional care settings. It must be recognized that not all caregivers are ready or equipped to properly care for older persons.
A caregiver may have a higher risk of committing elder abuse if they:
- Are highly financially or emotionally dependent on the elder
- Care for an elder with poor physical or mental health
- Have limited access to elder care services
- Have negative beliefs about aging and elders
- Have poor coping skills
- Lack social support
- Suffer from mental illness or substance abuse
- Lack training or proper preparation for caregiving responsibilities
- Were exposed to abuse as a child
The excessive stress and demands on caregivers can cause some to suffer from anxiety, depression, and psychological disorders. As anxiety and stress increase, caregivers may start to feel helpless and trapped in their situation. Individuals have different ways of relieving this stress, and some turn to substance abuse or abuse of their older family members.
The responsibilities and demands of caregiving increase as the older adult person’s condition deteriorates. Abused older persons are likely to have special problems, such as incontinence, shouting, wandering, or symptoms of paranoid delusions. Some traits prevalent among elders might be stubbornness, hypercritical attitudes, and somatization (psychological distress expressed as physical symptoms). These may represent attempts by the person to deal with a new dependency role and can be extremely difficult for caregivers to cope with, thus prompting abusive responses.
Respite care for the person and support groups and counseling for the caregiver can help to prevent elder abuse. In severe cases of abuse, it is usually necessary to separate the person from the caregiver, initiate legal action, and find a safe facility for the person.
Nursing home residents may be at higher risk for elder abuse in a facility with:
- Careless hiring practices, such as not doing thorough background checks
- High staff turnover rates
- Little administrative oversight
- Stressful working conditions
- Staff who act coldly or negatively toward residents
It is important to note that older patients residing in nursing homes are at risk of abuse not only from staff but also from other residents (Nursing Home Abuse Center, 2024; Piedmont Healthcare, 2024).
INTERVENING AND REPORTING ELDER ABUSE
Training on recognizing and reporting suspected elder abuse and neglect is critical to health professional education and patient safety. Such training should apply to all types of healthcare professionals, as each owes a legal duty to report these cases. Systemically, the chances of missing cases of abuse and neglect are minimized if all types of healthcare professionals have similar training regarding recognition of the issue.
Because older dementia patients usually cannot self-report instances of abuse, the responsibility for identification, intervention, and reporting rests largely with healthcare professionals, social service agencies, and police departments. Interventions vary from simple social service referral to the extreme of removing the person from the home.
Once suspected, elder abuse should be reported to the local Adult Protective Services (APS) agency. The National Center on Elder Abuse (NCEA) is a valuable tool in identifying state-specific resources to assist in reporting to the appropriate authorities.
Suspected abuse in long-term facilities should be reported to the state licensing agency. In some states, reports of abuse in facilities can be made to APS. Long-term care facilities include nursing homes, assisted living centers, and board and care facilities.
Elder abuse is defined by state laws, but state definitions vary considerably from one jurisdiction to another. They contain many sections regarding who is protected, who must report, definitions of reportable behavior, requirements for investigation of reports, penalties, and guardianship.
Every state has mandatory reporting laws in place specifically for elder abuse. Some laws and specifics may vary between states, such as how elder abuse is defined and when mandated reporters have to notify authorities. In addition to varying reporting laws, some states may have different agencies overseeing nursing home abuse.
Mandatory reporters are professionals who are designated by state statute to report incidents of suspected elder mistreatment to reporting agencies. Mandatory reporters may include healthcare professionals, social service providers, caregivers, clergy, and financial institutions, among others (Thomas & Reeves, 2023; NCEA, 2023).
(For more information on reporting abuse, see “Resources” at the end of this course.)
CASE
Mr. Moustaffa, a 72-year-old widower who lives alone, was seen in the dementia assessment unit after referrals by a concerned neighbor. The patient had previously been diagnosed with early dementia, but much of his conversation still made perfect sense. He repeatedly reported that his children “are ripping me off.” Per agency protocol, the unit social worker visited Mr. Moustaffa in his home to further assess his living situation.
During the visit, the social worker found that Mr. Moustaffa had written several large checks to his son for groceries in the past month, but that there was no food in the house. She learned that since Mr. Moustaffa was no longer able to drive, his son and daughter-in-law now did all the shopping for him, but that they gave him only a fraction of the groceries he was paying for and kept the rest of the money themselves. This information was used to assist in approaching the son and daughter-in-law with the concern of elder financial abuse.
LEARNING TO MANAGE PROBLEM BEHAVIORS
As Alzheimer’s disease progresses, dementia can cause mood swings and changes in the person’s personality and behaviors, including agitation and restlessness, vocal outbursts, wandering, sleep disturbances, “sundowning,” and inappropriate sexual activities. These behaviors can be very stressful for both the person with dementia and the caregivers. These challenges can be met by using creativity, flexibility, patience, and compassion. It also helps for caregivers to avoid taking things personally and to maintain a sense of humor.
Managing difficult behaviors effectively calls for special intervention training and education for staff and caregivers. Such training can help providers identify and anticipate problem behaviors and learn diversionary strategies to manage these behaviors. (See also “Resources” at the end of this course.)
Behavioral problems are major reasons why family caregivers decide to seek long-term care for their loved one. Facility staff can gain valuable insights from the family into the person’s behavioral history, which will aid in planning effective interventions. Together with psychological and medical evaluations, this behavioral history can alert staff to important triggers for behavioral problems.
DICE Tool for Problem Behaviors
DICE is a tool that can be used to help understand and respond to behaviors. It involves asking a series of questions in regard to the patient, caregiver, and environment, as summarized in the table below.
| (UCSF, 2024d) | ||
| D | Describe what happens |
|
|---|---|---|
| I | Investigate possible causes |
|
| C | Create a plan |
|
| E | Evaluate the plan |
|
Agitation and Aggression
Agitation is a state of extreme irritability often characterized by hitting, pacing, yelling, cursing, arguing, threatening, and verbal or physical aggression. This behavior often progresses with the stage of dementia, from mild to more severe. Agitation can be triggered by a number of things, including environmental factors, fear, fatigue, and feelings of abandonment. Often it is triggered when the person perceives that control is being taken away from them.
An agitated person requires an assessment for any physical cause of discomfort or pain. This can include fecal impaction, localized or systemic infection, dehydration, urinary retention, osteoarthritis, or fractures. The person may be hungry or thirsty or may be suffering from inadequate sleep. The following strategies may be helpful:
- Avoid confronting a confused person, as it may increase anxiety.
- Reduce noise, clutter, or number of persons in a room.
- Maintain structure by keeping the same routines; keep household objects and furniture in the same places.
- Reduce caffeine intake, sugar, and other foods that cause spikes in energy.
- Try gentle touch, soothing music, reading, or walks to quell agitation.
- Speak in a reassuring voice.
- Do not restrain the person during a period of agitation.
- Distract the person with a snack or an activity.
- Allow the person to do as much for themself as possible; support independence and ability to care for self.
- Validate the person’s feelings and then attempt distraction or redirection.
(FCA, 2024b)
Another approach to the problem of agitation is the three Rs: repeat, reassure, and redirect. Using this approach, the caregiver repeats an instruction or answer to a question, reassures the person, and redirects the person to a different activity to divert attention from the problem.
CASE
Mr. Hopkins is a 72-year-old male patient who was admitted to the nursing home three months ago because of his family’s inability to care for him at home any longer. He had been diagnosed with Alzheimer’s six years earlier. Each Sunday he became quite happy during visits from his family, but each time the family got ready to leave, Mr. Hopkins would become more agitated, follow them to the door, and attempt to leave with them. When staff tried to lead him back inside, he would become belligerent and combative. This behavior often resulted in his receiving a medication, which made him drowsy. At one point, a staff member was injured when she fell while trying to avoid Mr. Hopkins’ attempt to strike her.
Nursing home staff and family discussed his behavior and determined that Mr. Hopkins’ agitation may be due to feelings of abandonment. They devised a care plan in which the family would inform the staff 15 minutes before their intended time of departure from visits. The family planned to leave a small memento with Mr. Hopkins at each visit, and a staff member would then begin discussing the memento with him, encouraging some reminiscing. The family would say a quiet goodbye, and Mr. Hopkins would not be allowed to walk them to the door. The staff member would remain with him in his room for approximately 10 minutes after their departure.
This intervention appeared to distract Mr. Hopkins from his feelings of abandonment without changing the nature of the family visits. There were no further incidents of combative behavior from Mr. Hopkins.
Vocal Outbursts
Disruptive vocal outbursts—screaming, swearing, crying, shouting, negative comments to staff and/or other persons, self-talk—become increasingly common as Alzheimer’s progresses, confusion increases, and the ability to communicate is lost. Verbal outbursts are often triggered by fear, anger, depression, grief, confusion, helplessness, loneliness, sadness, impatience, and frustration. Environmental factors may include poor lighting, seasonal changes, overstimulation or lack of stimulation, loud noises, or excessive heat.
Outbursts may also signal physical illness or discomfort, including pain, hunger, thirst, incontinence, constipation, infection, or fatigue. Once the outburst has subsided, a thorough physical assessment may reveal an underlying physical problem, which can then be remedied.
Caregivers are encouraged to remember that the person is not deliberately misbehaving; these are not temper tantrums. React by staying calm and reassuring. Validate the person’s feelings and then try to distract or redirect the person’s attention to something else. Remarks or attacks should not be taken personally, nor should attempts be made to try to reason with the person.
Managing outbursts triggered by environmental or physical factors is simpler than dealing with outbursts that stem from an unknown emotional or psychological cause. Interventions to prevent verbal outbursts may include:
- Assess for pain, hunger, thirst, constipation, full bladder, fatigue, infections, and skin irritation.
- Avoid being confrontational or arguing about facts.
- Redirect the person’s attention.
- Remain flexible, patient, and supportive by responding to the emotion, not the behavior.
- Create a calm environment; avoid noise, glare, insecure spaces, and background distractions.
- Allow adequate rest between stimulating events.
- Provide the person with a security object.
- Acknowledge requests and respond to them.
- Look for reasons behind each behavior.
- Explore various solutions.
- Don’t take the behavior personally.
(Alzheimer’s Association, 2024l)
CASE
Mrs. Goh is a 78-year-old woman with Alzheimer’s disease who has been living in a nursing home for the past four years. She is known to have had a stroke resulting in expressive aphasia early in the disease process. Mr. Goh visits his wife daily at mealtimes and feeds her. He also reads to her while holding her hand until she falls asleep.
On his way to visit one day, Mr. Goh was involved in an accident and sustained a hip fracture. He was in the hospital for three weeks and then sent to a rehabilitation unit for short-term physical and occupational therapy. When Mrs. Goh was told about her husband’s accident, her condition began to decline. She became bedridden and uninvolved with any activities of daily living. She also began yelling and screaming for extended periods of time. This behavior became very disruptive to everyone. As her condition worsened, Mrs. Goh was moved to a private room, but it was in the front of the building, making her yelling audible to anyone entering the building.
The treatment team met to devise a plan of care. Their first step was a thorough assessment, with the following conclusions:
- Mrs. Goh’s current method of communication is screaming.
- She is reacting to the loss of her husband’s visits and other physical and social losses.
- She has discovered that screaming brings attention.
- By screaming she is able to exert some control over her life.
- The screaming occurs in the late afternoon, when she needs to use the toilet, or when she is overly fatigued.
- Previous tactics to control Mrs. Goh’s screaming have been ineffective.
After completing the assessment, the following plan was developed and implemented:
- No more changes are to be made in Mrs. Goh’s environment.
- Routines are to be established with Mrs. Goh’s input, and the same caregivers will provide her care on a daily basis to establish consistency in her daily life.
- The activities director will work with Mrs. Goh to add new activities.
- Arrangements are to be made for consistent volunteers to visit Mrs. Goh on a daily basis, attempting to establish a routine similar to her husband’s.
- Mrs. Goh will be given a bell to ring if she needs something. Otherwise, she will be checked on every two hours.
- Caregivers will be instructed to respond quickly to the bell but not to her screaming.
After two months, Mrs. Goh began using the bell to call for assistance. She still yells out occasionally, but this tends to occur when unavoidable changes are made to her routines.
Wandering
Any person that has memory problems and is able to walk is at risk for wandering and getting lost. If not found soon, wandering older persons with dementia may suffer serious injury or death.
Wandering occurs for a variety of reasons, such as boredom, medication side effects, or looking for something or someone. Agitation, restlessness, and sleep disturbances all lead to wandering, particularly at night, increasing the risk of injury to the person and others.
Wandering is generally one of two types: goal-directed, in which the person attempts to reach an impossible goal such as going home or going to the store, and non-goal-directed, in which the person wanders aimlessly. Wandering patterns include:
- Moving to a specific location
- Lapping or circling along a path or track
- Pacing back and forth
- Wandering at random
Discovering the triggers for wandering are not always easy, but they provide insight into the behavior. Wandering may represent a search for social interaction when the person can no longer communicate verbally. Unable to sleep, the person may walk to keep busy or to find a loved one. Wandering in the late afternoon or early evening may be triggered by a fading memory of leaving work to go home. Wandering may also be caused by a physical need, such as toileting.
The following techniques may be helpful when dealing with the issue of wandering in the home care setting.
- Make time for regular exercise to minimize restlessness.
- Use large-print signs to mark destinations with a drawing of the activity.
- Place a photo of the person as a younger adult on the room door to help the person find “home.”
- Ensure that doors have locks that require a key. Position them high or low on the door, as many people with dementia will look only at eye level. It is important to recognize that a danger of this approach is fire safety; the lock(s) must be accessible to others and not take more than a few seconds to open.
- Do not try to restrain the person unless there are obvious hazards, such as traffic or harsh weather.
- Try to remain calm and reassuring instead of controlling.
- Avoid negative or hard commands such as “Don’t go out there!”
- Avoid arguing with the person.
- Use a barrier, such as a curtain, to mask the door. A stop sign or “do not enter” sign may be effective.
- Paint a door to look like a piece of furniture.
- Paint a black space on the front porch that may appear to the person with dementia to be an impassable hole.
- Add “child-safe” plastic covers to doorknobs.
- Do not lock a person with dementia in the home or car unattended.
- Consider installing a home security system or monitoring system such as a GPS tracking device. (These may be effective only in areas with good cellphone coverage and in tandem with an attentive person monitoring the devices.)
- Put away items such as the person’s coat and purse; some people will not go out without taking certain articles with them.
- Sew ID labels in the person’s clothes or have the person wear an ID bracelet.
- Tell the neighbors about the person’s wandering behavior and provide them with a telephone number.
- Always have a current photo available should the need arise to report the person as missing.
Caregivers can also leave a copy of the person’s photo on file at the police department or register the person with the MedicAlert + Alzheimer’s Association Safe Return program, which is a nationwide emergency response service for individuals with Alzheimer’s or a related dementia. Registration includes an identification bracelet that should be worn at all times (FCA, 2021b). (See also “Resources” at the end of this course.)
When the person has been admitted to a care facility, the family can help staff identify and anticipate wandering. Family can inform the staff about the person’s lifestyle prior to being diagnosed with Alzheimer’s, which can aid in understanding behavior (i.e., what kind of work the person did; previous patterns of exercise, stress, and response to touch; etc.). Once a person who wanders is identified, the facility can have photographs made and distributed to other units and assign special clothing or identification bands. Facilities should consider painting all doors for staff only the same color as the wall, while doors the person is expected to find and use should contrast with walls.
Wandering in a safe area can be good exercise for the person with Alzheimer’s disease and help manage non-goal-directed wandering. Many facilities are designed with these safe areas in the form of sheltered courts, gardens, lounges, or pathways (Alzheimer’s Association, 2024l).
SILVER ALERT
Silver Alert programs inform law enforcement agencies, media outlets, and the public about missing adults, usually older adults with cognitive disabilities or impairments. The information that is distributed includes photographs, a vehicle description if the person was driving, last known location, home location, and medical condition.
Law enforcement agencies are most often responsible for deciding to activate a Silver Alert. Information is broadcast using dynamic message signs on roadways, radio stations, mobile phones, the internet, and television. Silver Alerts may also involve Reverse 911 or other emergency notification systems to alert nearby residents of the neighborhood surrounding the missing person’s last known location.
Silver Alert or Code Silver programs exist on a state-by-state basis. As a result, what incidents qualify for Silver Code may differ from state to state.
(CHP, 2024)
Sleep Issues and Sundowning
Restlessness, agitation, disorientation, and other troubling behavior in persons with dementia often worsen at the end of the day and sometimes continue through the night. This behavior is referred to as sundowning.
Possible contributing factors to sleep disturbance include:
- Mental and physical exhaustion from a full day trying to keep up with unfamiliar or confusing environment
- An upset in the biologic clock, causing a mix-up between day and night
- Reduced lighting that increases shadows, which may cause misinterpretation of what is seen and thereby increase agitation
- Stress due to noticing stress or frustration in other people around them.
- Disorientation due to inability to separate dreams from reality when sleeping
- Sleeping less, which is common among older adults
The following are some strategies to help manage sleep issues and sundowning:
- Schedule major activities in the morning or early afternoon hours when the person is most alert.
- Encourage a regular routine of waking up, eating meals, and going to bed.
- When possible and appropriate, include walks or time outside in the sunlight.
- Identify triggers that occur before sundowning events.
- Reduce stimulation during the evening hours, which may add to the person’s confusion.
- Offer a larger meal at lunch and keep the evening meal lighter.
- Keep the home well lit in the evening, which may reduce confusion.
- Do not physically restrain the person, as it can make agitation worse.
- Identify activities that are soothing, such as listening to music.
- Take the person for a walk to reduce restlessness.
- Discuss with the provider about best times of day for taking medications.
- Limit daytime naps.
- Reduce or avoid alcohol, caffeine, and nicotine, which can all affect ability to sleep.
If the person is awake and upset, approach in a calm manner, determine what the person may need, gently remind about the time, and offer reassurance that everything is all right. Avoid arguing, and allow the person to pace back and forth, as needed, with supervision (Alzheimer’s Association, 2024l).
CASE
Mrs. Perlman is a 72-year-old widow who was diagnosed with Alzheimer’s eight years ago and is now in the middle stage of the disease. She moved in with her daughter Jeanne about six months after being discharged from the hospital following treatment for pneumonia. Her level of confusion and disorientation has increased since her discharge.
Jeanne began attending a local Alzheimer’s support group once her mother moved in with her, and she has asked for help because her mother becomes “like another person after supper.” She says her mother no longer recognizes her, is disruptive, and can’t be calmed down until she falls asleep. Fortunately, her mother always seems much better in the morning.
The group asks questions to discover what can be done to help Jeanne with what many of them recognize as the problem of “sundowning.” They ask how a typical day goes, and Jeanne says her mother does not have an opportunity for a nap in the early afternoon, but she sleeps well at night. She says her mother is very hungry at suppertime, and since the fall daylight savings time change, her behavior has become worse.
Together they devise these methods to help Jeanne deal with her mother’s behavior:
- To avoid extreme fatigue, Jeanne has her mother take a one-hour nap at 1 p.m. but doesn’t allow her to sleep too long, since that may interfere with her sleep at night.
- To help relieve Mrs. Perlman’s hunger and possible low glucose level, Jeanne gives her mother a high-carbohydrate snack at 4 p.m.
- To maintain the same level of light in the house, Jeanne turns on all the lights two hours before sundown. She closes the curtains one hour before sundown so her mother might not notice the changing light level outside.
- Jeanne attempts to engage her mother in a quiet activity immediately after supper.
Two months later, Jeanne reports back to the support group that, although her mother still has some increased confusion at nighttime, the frequency and degree of confusion and disruption has decreased significantly.
Perseveration and Compulsive Behaviors
Repetitious speech or actions are those that occur on a continuous basis and generally serve no functional purpose. Mostly, these behaviors are tolerable for caregivers, but they can also become very annoying and lead to a great deal of frustration. These behaviors are due to the disease process and not because the person is purposely trying to be annoying. They may include:
- Checking locks, doors, or window coverings over and over
- Having rigid walking patterns, including pacing
- Collecting or hoarding items
- Counting or organizing objects repeatedly
- Going to the toilet frequently
- Selective eating habits
- Asking the same questions repeatedly
The caregiver can consider whether the person might have a need they are not able to express, such as boredom, hunger, insecurity, or need to use the toilet. Sometimes people engage in repetitive behavior because they are feeling anxious and the activity is soothing. It may be of benefit to see if there is a way to substitute the behavior with another activity, such as folding laundry, sweeping, or creative projects. Remove or hide objects in the environment that might trigger the behavior.
All behaviors have meaning. Repetitious activity often has a basis in the person’s past, such as work. A man who picks up the chairs, places them upside down onto a table, and wiggles their legs may be demonstrating a behavior required in his former work as a furniture maker or carpenter. A woman who worked in an office all her life may pace and exhibit restlessness. Simple measures such as dressing her in business attire and providing her with a small desk may prove to be a calming and reassuring activity. Other helpful measures to consider may include:
- Providing plenty of reassurance and comfort in word and touch
- Distracting with a snack or activity
- Avoiding reminding the person that they just asked the same question
- Ignoring the behavior or question, and instead refocusing the person into an activity such as singing or helping with a chore
- Not discussing plans with a confused person until immediately prior to an event
- Learning to recognize certain behaviors (e.g., an agitated state or pulling at clothing to indicate a need to use the bathroom)
When the person is very rigid and resistant to any interference with the activity, it is important to avoid provoking an aggressive reaction.
- Use a calm, matter-of-fact tone of voice.
- Do not become bossy or condescending.
- Distract the person with something appealing to them.
(UCSF, 2024d; FCA, 2024b)
Shadowing is another repetitive behavior in which the person constantly follows their caregivers around. This behavior often occurs late in the day. They may imitate the caregiver or become anxious if the caregiver tries to spend any time away from them. For the caregiver, this can be a smothering experience. Even being able to use the bathroom alone can be a challenge. In Alzheimer’s patients, shadowing represents a message of uncertainty, insecurity, or fear. Caregivers represent security and protection. Helpful suggestions include:
- Establish and maintain a daily routine to help the person feel more secure.
- Say reassuring words every day and often, such as, “You’re safe.”
- Avoid moving household furnishings or other items around or rearranging them.
- Use a simple whiteboard to indicate the date or to tell the person when the caregiver will return.
- Involve and engage the person in familiar activities such as folding laundry or cleaning tasks.
- Play the person’s favorite musical selections.
- Make an audiotape of the caregiver’s voice or any reassuring familiar voice.
- Make a videotape of recent events to play for the person, or play familiar movies.
- Consider the use of a day center or hiring a professional caregiver.
(Alzheimer’s Association, 2024l)
Inappropriate Sexual Behaviors
Because of dementia, many individuals lose the ability to determine the appropriate time, place, or way to express sexual needs. Inappropriate behaviors may become the only available mechanism for gratifying such needs. Acts of sexual disinhibition result from damage mainly in the frontal and temporal lobes of the brain, disrupting the person’s ability to control behaviors.
Such behaviors may include masturbation, undressing in public, and making lewd remarks or unreasonable sexual demands, as well as sexual aggression, which may include fondling, exposing genitals, or attempting to engage in sex acts with people other than their partners. This behavior may be directed toward their own children, professional caregivers, or others because of the person’s inability to recognize the individual is not their partner.
Persons who masturbate in public places should be gently led from the public area to their room. Do not scold or try to get them to understand the inappropriateness of their behavior, as that will only increase their negative feelings and agitation.
If possible, identify what is triggering the behavior. Every attempt should be made to determine whether the person is suffering from pruritus, an infection, or a chronic stress condition. Assess behaviors for any antecedent events such as a visit from the family. If persons have truly problematic sexual behaviors such as touching visitors or staff persons intimately following a family visit, for instance, visitation should take place in the person’s room, and once the family leaves, the person should immediately be involved in some activity.
Undressing in public may be due to physical factors such as being too warm or frustration about trying to remember how to dress and undress. Specially designed clothing that closes in the back makes disrobing difficult in inappropriate settings.
Forewarn family and friends of the person’s behaviors to better prepare them with what to expect and how best to respond. Because of the embarrassment and negative feelings about these behaviors, family members, friends, and caregivers must be given an opportunity to talk about their feelings.
If the person is disruptive or making someone else uncomfortable, make eye contact and say, “Stop,” with a calm but firm tone of voice, and then distract with a different activity.
If it is thought that the person is seeking more physical affection or intimacy, consider pet therapy, a stuffed animal, and socially appropriate touching such as hand-holding, dancing, back rubs or massages, manicure/pedicure, or brushing/combing hair (UCSF, 2024d).
CARING FOR THE CAREGIVERS
The role of caregiving often falls to a family member. Approximately two thirds of dementia caregivers are women, one third are age 65 or older, and one quarter are “sandwich generation” caregivers, meaning that they care not only for an aging parent but also for children under age 18. As the disease progresses, care needs become greater, requiring more hours of the caregiver’s time, and the more hours the caregiver devotes, the higher the risk of caregiver overload and stress-related health issues (CDC, 2023).
The Effects on Caregivers
Caregivers are often referred to as hidden victims because they commonly experience more psychological and health problems than those who are not caregivers. Evidence shows that most caregivers are poorly prepared for their role and provide care with little or no support. Family members who provide care to individuals with dementia or other chronic conditions are themselves at risk. Emotional, mental, and physical health problems arise from complex caregiving situations and the strains of providing care.
Higher levels of stress, anxiety, depression, and other mental health effects are common among caregivers. Depression and anxiety disorders in caregivers persist and can even worse after placement of the patient in a nursing home. Many caregivers report experiencing symptoms such as:
- High levels of stress and feeling frustrated, drained, angry, guilty, and helplessness
- A loss of self-identity, lower levels of self-esteem
- Constant worry
- Feelings of uncertainty
Research has shown that female caregivers (about two thirds of all unpaid caregivers) fare worse than male caregivers, reporting higher levels of depression and anxiety, lower levels of subjective well-being, less satisfaction with life, and poorer physical health.
Caregivers who experience chronic stress may be at greater risk for their own cognitive decline, including loss in short-term memory, attention, and verbal IQ. Studies have found that caregivers have diminished immune response, leading to frequent infections and increased risk of cancers. Older spousal caregivers (ages 66–96) who experience caregiving-related stress have a 63% higher mortality rate than noncaregivers of the same age, and hospitalization of an older spouse has been found to be associated with an increased risk of caregiver death (FCA, 2024c).
In response to increasing stress, caregivers have been found to have increased alcohol and other substance use, and to use prescription and psychotropic drugs more than noncaregivers. Spousal caregivers who are at risk of clinical depression are more likely to engage in harmful behavior toward the person.
Keeping family caregivers healthy and able to provide care is important to the nation’s long-term care system, and with the aging of the population, this will become more important in the future (FCA, 2024c).
Strategies to Manage Caregiver Stress
Although caregiving can have a negative impact on the caregivers’ health and well-being, research demonstrates its effects can be alleviated at least in part by:
- A family physician assessment of caregiver needs that leads to a care plan with support services, and repeat assessments with changes in status of caregiver or care recipient
- Caregiver education and support programs, including national caregiving organizations, local elder care agencies, and websites (see also “Resources” at the end of this course)
- Respite care to reduce caregiver burden, such as in-home, adult care centers and programs or short-term nursing homes
- Financial support to alleviate the economic stress of caregiving
- Primary care interventions that address caregiver needs
(FCA, 2024c)
Compensation for Family Caregivers
When a family member becomes a caregiver, it may impact family finances by requiring the caregiver to make adjustments in employment status. In the United States, it is possible for a caregiver to be paid for caring for a family member with dementia. There are a variety of different programs that offer this option; however, there can be significant hurdles in the process of taking advantage of them.
Governmental programs that pay family members are offered in most, but not all, states. These programs include:
- Medicaid Home and Community-Based Services Waivers (HCBS) provide financial assistance to purchase home and community-based services and supports. These waivers allow the person with dementia to choose whom they wish to be a personal caregiver, which could be a family member.
- Medicaid state plans offer personal care services. Often states allow family members to be personal care providers and offer cash compensation.
- Adult foster care through Medicaid allows an individual with dementia to move into the home of a caregiver to receive around-the-clock supervision. These programs provide compensation for providing care but do not provide cost of room and board.
- The Caregiver Child Exemption is a Medicaid exemption for an adult child who has a parent with dementia. It is similar to foster care; however, the adult child moves into the home of the parent to provide care. Medicaid rules usually require that the home be forfeited in exchange for care, allowing the home to be transferred to the adult child for compensation after residing in and providing care for their parent for a minimum of two years.
Thirteen states and the District of Columbia have established a comprehensive mandatory state paid family leave system. Other programs are available for veterans (Dementia Care Central, 2023b).
ETHICAL AND END-OF-LIFE CONSIDERATIONS
Alzheimer’s disease raises a host of ethical issues. Such issues can be addressed by considering these three guiding ethical principles that are commonly applied in patient care:
- Beneficence: the obligation to do good, preserve life, and prevent harm and suffering
- Respect: the obligation to preserve and promote the autonomy and dignity of the person
- Equity: the obligation to give treatment that is fairly and equitably distributed among individuals
The obligation to evaluate and treat physical illness is not diminished by the person’s age or mental state. Ethical principles ensure a person’s right to adequate treatment for preservation of life or prevention of suffering.
Decision-Making
It is not uncommon for caregivers to behave paternalistically toward the person with dementia. However, persons with dementia should be given every opportunity to play a role in decision-making, even if only about minor aspects of their environment. Some persons are capable of making many decisions, and others, none. This requires an ongoing individualized assessment that is also periodically conducted in a more formal fashion to ascertain level of competence. Respecting the decision-making capacity of each person helps prevent steadily increasing dependence.
Individuals are presumed capable of acting in their own best interest, and a healthy and competent adult has the legal and moral right to choose and refuse. It is this major right to make such choices that is at issue in Alzheimer’s disease.
It is imperative that decision-making and preferences about medical treatment begin early in the disease process through execution of advance directives. In the absence of an advance directive, the surrogate decision maker should be guided by the values and expressed wishes of the person with Alzheimer’s.
Questions that individuals may wish to consider when making decisions about medical treatment at the end of life include:
- In what type of environment do I want to spend the end of my life (e.g., at home, in a nursing facility)?
- What are my religious, spiritual, or culture beliefs about end of life, and how can these be respected and honored?
- Do I want all available treatment measures to be taken? Are there any treatments I do not want?
LIFE-EXTENDING TREATMENT
In the United States, the basic right to be free from unwanted treatment is long established. People with decision-making capacity have the right to forgo life-sustaining treatment they do not want, and people with dementia have the same right through the use of advanced directives or a surrogate.
Ethical dilemmas often occur when decisions are being made regarding end-of-life issues such as withholding treatment and “letting nature take its course.” The person’s wishes, if known and expressed while still competent, should be considered. Persons have the right to refuse life-extending treatment, and incompetence does not diminish that right. When a person with Alzheimer’s disease, family members, and the caregiver cannot agree on these matters, the decisions must be left to the courts.
People with advanced dementia who have a valid advance directive most often document a preference for supportive care at the end of life. It has also been shown that people who have an advance directive are less likely to have burdensome treatments at the end of life, including feeding tubes, hospitalizations, and intensive care unit stays in their last months of life (Compassion & Choices, 2024).
Aggressive medical care that will sustain life may include:
- Respirators
- Feeding tubes
- IV hydration
- Antibiotics
- Cardiopulmonary resuscitation (CPR)
- Surgery
For those who do not wish to be resuscitated, a do-not-resuscitate (DNR) order can be obtained (see below).
AID IN DYING
Medical aid in dying is a legal practice in 11 jurisdictions in the United States, including:
- California
- Colorado
- District of Columbia
- Hawaii
- Maine
- Montana
- New Jersey
- New Mexico
- Oregon
- Vermont
- Washington
In some states it is legal for mentally competent adults (ages 18 and over) with a terminal illness and a prognosis of 6 months or fewer to decide for themselves what a good death means in accord with their state’s aid-in-dying laws. However, persons with Alzheimer’s disease/dementia are ineligible to request life-ending medications under such “death with dignity” laws because their judgment and decision-making are impaired by their illness.
A person must be mentally competent and be capable of making their own healthcare decisions, and when making requests for medications under aid-in-dying laws, the person must be able to self-administer and ingest the medication at the time of their choosing. This, of course, also makes the Alzheimer’s patient ineligible. Also, if a person is in the early stages of the illness without cognitive impairment and does not have another disease that is causing a terminal illness with 6 months or less to live, they do not qualify to make that request (Death with Dignity, 2023; Treem, 2023).
ARTIFICIAL NUTRITION AND HYDRATION (ANH)
People living with dementia decline in their ability to eat and drink as symptoms progress. These include dysphagia, loss of appetite, inability to recognize food and utensils, difficulties with attention, and problems maintaining an eating routine. Decreased oral intake in the advanced stages of the disease is not believed to cause discomfort for the person living with dementia; however, family members and professionals may feel obligated to continue feeding to avoid feeling they are being neglectful.
ANH is sometimes offered via invasive procedures such as a nasogastric tube, percutaneous endoscopic gastrostomy (PEG) tube, intravenous hydration, and hypodermoclysis (subcutaneous fluid infusion). The evidence of the effectiveness of these interventions is limited, and they can have a negative effect on the well-being of the person with dementia.
Tube feeding has been found to be associated with increased mortality rate and possible tube-related complications, including a higher risk of pneumonia (Lee et al., 2021).
Legal Documents
Getting legal affairs in order—drawing up advance directives, powers of attorney, wills, or trusts—should be done as soon as possible after diagnosis, while the person is able to express personal wishes and participate in decisions. Referral to the local chapter of the Alzheimer’s Association can help families find attorneys who specialize in elder law or estate planning.
This referral should not be made abruptly but as a suggestion, emphasizing that every adult, regardless of health status, should make such a plan. This helps ensure that one’s wishes are respected in end-of-life care and disposition of property after death. Otherwise, families will have to make difficult decisions without knowing the person’s wishes.
ADVANCE DIRECTIVES
An advance directive, also known as a living will, allows the person to document their wishes concerning medical treatments at the end of life in the event they are unable to communicate those wishes. The advance directive never expires, remaining in effect until the person changes it. Every new advance directive invalidates the previous one. In the advance directive, the person can name a representative to ensure wishes concerning care are carried out. This representative is called a durable power of attorney for healthcare or medical power of attorney.
It is important to note that emergency medical technicians cannot honor an advance directive. The only document they honor is the do-not-resuscitate (DNR) order (see below).
Physicians should have copies of advance directives available or be able to refer families to a source for the appropriate forms. Federal law requires hospitals to inform patients that they have a right to complete an advance directive, but advance directives are regulated by state law and may differ from state to state. (See also “Resources” at the end of this course.)
Three specific advance directives have been developed by some states in the United States to provide a measure of control to those with dementia, with the aim of helping them make their intentions known in case they are no longer able to communicate. Alzheimer’s directives are documents that help the patient’s family and caregivers know their preferences for healthcare. However, they may not be legally binding outside the states where they were developed. These include:
- Living with Dementia Mental Health Advance Directive
- Health Directive for Dementia
- Advance Directive for Receiving Oral Food and Fluids in the Event of Dementia
These directives provide a means for those diagnosed with dementia, while still retaining their decision-making capacity, to limit assisted feeding by hand when they reach the final/terminal stage of the disease. The instructions in the directive become effective when the person’s appointed health care agent, in concert with the primary care physician, agrees that the patient is now in the final stage of dementia and is unable to make treatment decisions or to self-feed.
Unlike the standard advance healthcare directive, which specifies what medical actions should be undertaken if the person is too ill or incapacitated to make decisions, an Alzheimer’s/dementia directive covers decisions involving day-to-day choices such as where the person would like to be treated, who the preferred caregiver is, and who’s authorized to be the person’s healthcare agent (Death with Dignity, 2024).
DO-NOT-RESUSCITATE ORDERS
Another type of advance directive is a do-not-resuscitate order (DNR or DNRO), which informs medical personnel that a person does not want to have cardiopulmonary resuscitation (CPR) performed in the event of cardiac or respiratory arrest. These orders are regulated by state law. (In 2005, the American Heart Associated moved from the traditional term DNR to “do not attempt resuscitation” [DNAR]. However, most facilities continue to use the term DNR.)
A DNR order should be posted prominently to ensure that the patient’s wishes will be honored in the event of an emergency. Emergency medical personnel do not have time to search for a DNR order, so it should be placed in an easily identifiable area. If the patient is in an institution, it should be prominently placed in the medical file. If the person is at home, it should be placed somewhere where paramedics or other EMS personnel can easily see it, such as taped to the foot of the individual’s bed or on the wall near the bed. Some people prefer the additional safeguard of wearing a bracelet or necklace to alert care providers that a DNR order is in force (Eisenhower Health, 2024).
Palliative Care and Hospice Care
Palliative care provides relief from distressing symptoms, including pain, shortness of breath, nausea, insomnia, and side effects of medications. People usually receive palliative care at clinics or hospitals, but home care may be possible. It can be provided at any point during the disease process as needed.
Hospice seeks to optimize the quality of life in the terminally ill (typically, those expected to live six months or less), while neither hindering nor hastening the dying process. The hospice philosophy emphasizes physical comfort, pain and symptom management, and death with dignity. It encompasses the spiritual and psychosocial aspects of care, both for the patient and for the family, and includes bereavement support for the surviving family members. Hospice care involves a team of health professionals, including doctors, nurses, social workers, clergy, therapists, and trained volunteers.
During the terminal stages of Alzheimer’s, hospice care can be particularly beneficial to patients and families. Hospice care can be provided in the patient’s home, assisted-living residences, nursing homes, or specifically designated hospice care facilities. Medicare is the primary source of payment for hospice care, but private insurance, Medicaid, and other resources also will pay for it.
To qualify for insurance reimbursement (including Medicare) for hospice services, a physician and a hospice medical director must certify that the patient has less than 6 months to live. The National Hospice and Palliative Care Organization has published guidelines to identify which dementia patients are likely to have a prognosis of six months or less if the disease runs its normal course. Medicare covers the cost of hospice care in every state, as does most private long-term care insurance.
Physicians and other health professionals educate families about the benefits of hospice care for their loved one with Alzheimer’s disease and for themselves. Ideally, this education begins at the time of diagnosis, when the person is still capable of expressing preferences about end-of-life care (Death with Dignity, 2024).
CONCLUSION
Alzheimer’s disease is one of the most devastating conditions that affects human beings because it destroys the mind. Dementia impairs memory and interferes with the ability to make rational decisions, thus preventing persons from functioning effectively in their environment. As a result, dementia robs the person of dignity and independence. Because Alzheimer’s disease is completely irreversible, cannot yet be adequately treated, and is often associated with a long survival period, it affects not only the person’s life, but also the person’s family, caregivers, and society. For caregivers, the challenges can be overwhelming. It is essential that professionals recognize the toll this disease takes on both the person with the disease and those who are charged with their care.
The risk of developing Alzheimer’s dementia increases with age, and as the population continues to age, the number of persons with Alzheimer’s disease will also increase. Along with this, the need for caregivers and support for them will be greater than ever. Healthcare professionals must become educated about this disease and its impact in order to be aware of the ways in which they can effectively care for these individuals and their caregivers. They must understand the impact of this disease on the person and learn how to use effective strategies to manage difficult behaviors. It is important for healthcare professionals to use a person-centered approach, which involves developing a therapeutic relationship and getting to know the person’s life story and preferences so as to provide the quality of care they deserve.
RESOURCES
Alzheimer’s disease (MedlinePlus)
Caregiver training videos (UCLA Health)
Financial assistance for family caregivers
Preventing Alzheimer’s disease and dementia (HelpGuide)
State elder abuse statutes (U.S. DOJ)
Wandering (Alzheimer’s Association)
REFERENCES
NOTE: Complete URLs for references retrieved from online sources are provided in the PDF of this course.
AbilityLab. (2024). Occupational therapy assessment tools. http://www.sralab.org
Alzheimer’s Association (AA). (2024a). 2024 Alzheimer’s disease facts and figures. https://www.alz.org
Alzheimer’s Association (AA). (2024b). Milestones. https://www.alz.org
Alzheimer’s Association (AA). (2024c). Prevalence of Alzheimer’s disease dementia in the 50 U.S. states. https://alz-journals.onlinelibrary.wiley.com
Alzheimer’s Association (AA). (2024d). Facts about race, ethnicity and Alzheimer’s disease. https://www.alz.org
Alzheimer’s Association (AA). (2024e). Native Americans and Alzheimer’s. https://www.alz.org
Alzheimer’s Association (AA). (2024f). Traumatic brain injury. https://www.alz.org
Alzheimer’s Association (AA). (2024g). Can Alzheimer’s disease be prevented? https://www.alz.org
Alzheimer’s Association (AA). (2024h). 10 early signs and symptoms of Alzheimer’s. https://www.alz.org
Alzheimer’s Association (AA). (2024i). Medical tests for diagnosing Alzheimer’s dementia. https://www.alz.org
Alzheimer’s Association (AA). (2024j). Earlier diagnosis. https://www.alz.org
Alzheimer’s Association (AA). (2024k). Medications for memory, cognition and dementia-related behaviors. https://www.alz.org
Alzheimer’s Association (AA). (2024l). Treatments for behavior. https://www.alz.org
Alzheimer’s Association (AA). (2024m). Communication and Alzheimer’s. https://www.alz.org
Alzheimer’s Association (AA). (2024n). Daily care plan. https://www.alz.org
Alzheimer’s Association (AA). (2024o). Medication safety. https://www.alz.org
Alzheimer’s Association (AA). (2023a). 2023 Alzheimer’s disease facts and figures. https://doi.org/10.1002/alz.13016
Alzheimer’s Association (AA). (2023b). Diabetes and cognitive decline. https://www.alz.org
Alzheimer’s Association (AA). (2021). Alzheimer’s and dementia. https://www.alz.org
Alzheimer’s New Zealand. (2024). What happens in the later stages of dementia. https://alzheimers.org
Alzheimer’s Research Association (ALZRA). (2024). Does smoking increase the risk of dementia? https://alzra.org
Alzheimer’s Society. (2024a). About dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024b). Why is dementia different for women? https://www.alzheimers.org
Alzheimer’s Society. (2024c). Hearing loss and the risk of dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024d). Depression and dementia risk. https://www.alzheimers.org
Alzheimer’s Society. (2024e). Obesity and dementia risk. https://www.alzheimers.org
Alzheimer’s Society. (2024f). Physical activity and the risk of dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024g). Social isolation and dementia risk. https://www.alzheimers.org
Alzheimer’s Society. (2024h). Brain training and dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024i). Sight and hearing loss with dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024j). Depression and dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024k). Understanding and supporting a person with dementia. https://www.alzheimers.org
Alzheimer’s Society. (2024l). Non-verbal communication and dementia. https://www.alzheimers.org
Alzheimer’s Society. (2023a). Hormones and dementia risk. https://www.alzheimers.org
Alzheimer’s Society. (2023b). Sleep and the risk of dementia. https://www.alzheimers.org
Alzheimer’s Society. (2023c). Time-shifting and dementia. https://www.alzheimers.org
Alzheimer’s Society of Canada. (2024). Positive approach to care (PAC). https://alzheimer.ca
American Occupational Therapy Association (AOTA). (2024). OT home health evaluation checklist & quality measures. https://www.aota.org
American Speech-Language-Hearing Association (ASHA). (2024). Dementia. https://www.asha.org
Barkley C. (2023). Several vaccines associated with reduced risk of Alzheimer’s disease in adults 65 and older. https://www.uth.edu
Biogen. (2024). Biogen to realign resources for AD’s franchise. https://investors.biogen.com
Blanchard C. (2024). The five “A’s” of Alzheimer’s. https://www.alztennessee.org
Boltz M, Kuzmik A, Resnick B, et al. (2023). Delirium and behavioral symptoms in persons with dementia at hospital admission: Mediating factors. Alzheimer Dis Assoc Disord, 37(2), 120–7.
Breakthru Physical Therapy Fitness. (2024). Alzheimer’s care. https://www.breakthruptfitness.com
Brockmann R, Nixon J, Love B, & Yunusa I. (2023). Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: Patient outcomes, healthcare costs, and drug development. Lancet Reg Health Am, 20, 100467. https://doi.org/10.1016/j.lana.2023.100467
Brookshire B. (2024). 5 reasons we don’t have a cure for Alzheimer’s disease. AARP. https://www.aarp.org
California Highway Patrol (CHP). (2024). Silver Alert. https://www.chp.ca.gov
Centers for Disease Control and Prevention (CDC). (2023). Caregiving for a person with Alzheimer’s disease or a related dementia. https://www.cdc.gov
Centers for Disease Control and Prevention (CDC). (2022). Down syndrome and increased risk for Alzheimer’s. https://www.cdc.gov
Çevik B, Kav S, Çıtak EA, Akyüz E, & Abbasoğlu A. (2022). Challenges of providing nursing care to patients with dementia: A qualitative study. Eur J Geriatric Gerontol, 3, 205–11.
Cimons M. (2023). Can vaccinations save your brain? AARP. https://www.aarp.org
Compassion and Choices. (2024). Dementia and end-of-life care. https://compassionandchoices.org
Coyne R. (2021). The Lawton scale for IADLs. https://hign.org
Dave D. (2023). How does the environment impact dementia care? https://www.sondercare.com
Death with Dignity. (2023). Medical aid in dying as an end-of-life option offers death with dignity. https://deathwithdignity.org
Dementia Care Central. (2024). Mini-mental state exam (MMSE) Alzheimer’s / dementia test: Administration, accuracy and scoring. https://www.dementiacarecentral.com
Dementia Care Central. (2023a). Balance problems associated with Alzheimer’s & dementia: Causes & solutions. https://www.dementiacarecentral.com
Dementia Care Central. (2023b). Medicaid benefits for dementia & Alzheimer’s care: At home in memory care & nursing homes. https://www.dementiacarecentral.com
Dementia Care Notes. (2024). Help with activities of daily living. https://dementiacarenotes.in/caregivers/toolkit/adl/
DePlano L, Saitta A, Oddo S, & Caccamo A. (2024). Epigenetic changes in Alzheimer’s disease: DNA methylation and histone modification. Cells, 13(8), 719. https://doi.org/10.3390/cells13080719
Eisenhower Health. (2024). Advance directive DNR for EMS. https://eisenhowerhealth.org
Encyclopedia of Mental Disorders. (2024). Cognistat. http://www.minddisorders.com
Family Caregiver Alliance (FCA). (2024a). Dementia and driving. https://www.ca
Family Caregiver Alliance (FCA). (2024b). Caregiver’s guide to understanding dementia behaviors. https://www.ca
Family Caregiver Alliance (FCA). (2024c). Caregiver health. https://www.ca
Gasnick K. (2024). Types of apraxia and how they are treated. VeryWellHealth. https://www.verywellhealth.com
Ghahremani M, Smith E, et al. (2023). Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. https://alz-Journals.onlinelibrary.wiley.com
Global Times. (2023). Incidence of Alzheimer’s disease in China becoming younger and younger, report shows. https://www.globaltimes.cn/page/202309/1298638.shtml
Hamilton J. (2024). New blood tests can help diagnose Alzheimer’s. Are doctors ready for what’s next? https://www.npr.org
Heerema E. (2024). Validation therapy for people with dementia. VeryWellHealth. https://www.verywellhealth.com
Heerema E. (2022a). 4 types of memory: Sensory, short-term, working & long-term. VeryWellHealth. https://www.verywellhealth.com
Heerema E. (2022b). Using reality orientation in Alzheimer’s and dementia. VeryWellHealth. https://www.verywellhealth.com
Hsu A. (2023). Flu shot may lower risk of Alzheimer’s disease. https://mydoctor.kaiserpermanente.org
Huang W, Huang J, Huang N, & Luo Y. (2023). The role of TREM2 in Alzheimer’s disease: From the perspective of tau. Front Cell Dev Biol, 11, 1280257. https://doi.org/10.3389/fcell.2023.1280257
Iowa GeriatricPain.org. (2024). Pain communication tools. https://geriatricpain.org
Jiao B, Rihui L, Zhou H, et al. (2023). Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimers Res Therapy, 15, 32. https://doi.org/10.1186/s13195-023-01181-1
Johns Hopkins Medicine. (2024). Stages of Alzheimer’s disease. https://www.hopkinsmedicine.org
Johnson R, Tolan D, Bredesen D, Nagel M, et al. (2023). Could Alzheimer’s disease be a maladaptation of an evolutionary survival pathway mediated by intracerebral fructose and uric acid metabolism? American Journal of Clinical Nutrition, 117(3), 455–66.
Karrer M, Zeller A, & Mayer H. (2022). Dementia care in acute hospitals: A framework for practice development and they-based evaluation. https://onlinelibrary.wiley.com
Keuning-Plantinga A, Roodbol P, van Munster B, & Finnema E. (2021). Experiences of informal caregivers of people with dementia with nursing care in acute hospitals: A descriptive mixed-methods stay. J Adv Nurs, 77(12), 4887–99.
Kim N & Lee H. (2021). Stereoscopic depth perception and visuospatial dysfunction in Alzheimer’s disease. Healthcare, 9, 157. https://doi.org/10.3390/healthcare9020157
Kumar A, Sidhu J, et al. (2024). Alzheimer’s disease. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
LaFee S. (2021). Blanks for the memory. https://health.ucsd.edu
Lakhan S. (2024). Alzheimer disease workup. Medscape. https://emedicine.medscape.com
Lanctôt K, Hahn-Pedersen H, Eichinger C, et al. (2023). Burden of illness in people with Alzheimer’s disease: A systematic review of epidemiology, comorbidities and mortality. J Prev Alzheimer’s Dis, 11(1), 97–107. https://doi.org/10.14283/jpad.2023.61
Lee Y, Hsu T, Liang C, Yeh T, et al. (2021). The efficacy and safety of tube feeding in advanced dementia patients: A systemic review and meta-analysis study. JAMDA, 22(2), 357–63. https://doi.org/10.1016/j.jamda.2020.06.035
Levi S, Ripamonti M, Moro A, & Cozzi A. (2024). Iron imbalance in neurodegeneration. Molecular Psychiatry, 29, 1139–52.
Li X, Zhang Y, Zhang C, et al. (2023). Education counteracts the genetic risk of Alzheimer’s disease without an interaction effect. Front Public Health, 11, 1178017.
Lv B, Liang L, Chen A, Yang H, et al. (2023). Mortality of Alzheimer’s disease and other dementias in China: Past and future decades. Int J Public Health. https://www.ncbi.nlm.nih.gov
Mayo Clinic. (2024). Diagnosing Alzheimer’s: How Alzheimer’s is diagnosed. https://www.mayoclinic.org
Mayo Clinic. (2023). Alzheimer’s stages: How the disease progresses. https://www.mayoclinic.org
McDermott KB & Roediger HL. (2024). Memory (encoding, storage, retrieval). In R Biswas-Diener & E Diener (Eds.), Noba textbook series: Psychology. DEF Publishers. https://nobaproject.com
MD+CALC. (2024). Barthel index for activities of daily living (ADL). https://www.mdcalc.com
Medicare Interactive. (2024). Items and services excluded from Medicare coverage. https://www.medicareinteractive.org
Mehta R & Mehta R. (2023). The vascular-immune hypothesis of Alzheimer’s disease. Biomedicines, 11(2), 408. https://doi.org/10.3390/biomedicines11020408
Miller J. (2022). Study finds some of the world’s lowest dementia rates in Amazonian indigenous groups. https://today.usc.edu
National Center on Elder Abuse (NCEA). (2024). Prevalence of elder mistreatment. https://ncea.acl.gov
National Center on Elder Abuse (NCEA). (2023). Elder justice. https://ncea.acl.gov
National Council on Aging (NCOA). (2024a). Elder abuse nursing home abuse center. Elder abuse prevention. https://www.nursinghomeabusecenter.com
National Council on Aging (NCOA). (2024b). Get the facts on elder abuse. https://www.ncoa.org
National Health Service (NHS). (2024). Activities for dementia. https://www.nhs.uk/conditions/dementia/living-with-dementia/activities
National Institute on Aging (NIA). (2024a). What causes Alzheimer’s disease? https://www.nia.nih.gov
National Institute on Aging (NIA). (2024b). Communicating with someone who has Alzheimer’s disease. https://www.nia.nih.gov
National Institute on Aging (NIA). (2023a). Alzheimer’s disease fact sheet. https://www.nia.nih.gov
News-Medical.Net. (2024). Study identifies a urinary biomarker to reveal early-stage Alzheimer's disease. https://www.news-medical.net
Nursing Home Abuse Center. (2024). Protecting seniors from abuse and neglect. https://www.nursinghomeabusecenter.com
Oregon Pain Guidance (OPG). (2024). Pain control in the elderly and individuals with dementia. https://www.oregonpainguidance.org
Penn Medicine. (2023). Individuals with depression are over two times as likely to be diagnosed with dementia, Penn Medicine research finds. https://www.pennmedicine.org
Physiopedia. (2024a). Activities of daily living. https://www.physio-pedia.com
Physiopedia. (2024b). Alzheimer’s disease. https://www.physio-pedia.com
Physiopedia. (2024c). Tinetti test. https://www.physio-pedia.com
Piedmont Healthcare. (2024). Dementia caregivers guide. https://www.piedmont.org
Pizzi L, Prioli K, Jukowitz E, Piersol C, et al. (2023). Economic analysis of the Tailored Activity Program: A nonpharmacological approach to improve quality of life in people living with dementia and their caregivers. J Appl Gerontol, 42(7), 1433–44.
Press D. (2021). Management of neuropsychiatric symptoms of dementia. UpToDate. https://www.uptodate.com
Press D & Buss S. (2024). Treatment of Alzheimer disease. UpToDate. https://www.uptodate.com
Reed-Geaghan E. (2022). Why does Alzheimer’s affect more women than men? https://www.brightfocus.org
Robinson E. (2023). OHSU scientists discover new cause of Alzheimer’s, vascular dementia. https://news.ohsu.edu
Rosenzweig A. (2024). Montreal Cognitive Assessment (MoCA) test for dementia. VeryWellHealth. https://www.verywellhealth.com
Saeed A, Lopez O, & Reis S. (2023). Cardiovascular disease: The heart-brain axis 2023. Journal of the American Heart Association, 12(21). https://doi.org/10.1161/JAHA.123.030780
Saragih D, Tonapa S, Yao C, et al. (2022). Effects of reminiscence therapy in people with dementia: A systematic review and meta-analysis. J Psychiatr Ment Health Nurse, 29(6), 883–903. https://doi.org/10.1111/jpm.12830
Schneider A, Moon C, Whitaker K, et al. (2022). Association of sleep with risk of Alzheimer’s disease mortality: NIH-AARP Diet and Health Study. J Appl Gerontol, 41(4), 1057–65. https://doi.org/10.1177/07334648211019207
Shukla D. (2023). Could fructose contribute to the development of Alzheimer’s? https://www.medicalnewstoday.com
Shuman T & Altman A. (2024). Validation therapy in dementia care. https://www.seniorliving.org
Simply Psychology. (2022). Procedural memory: Definition, examples, and how it works. https://www.simplypsychology.org
Skaria A. (2022). The economic and societal burden of Alzheimer’s disease: Managed care considerations. Am J Manag Care, 28(suppl 10), S188–96. https://doi.org/10.37765/ajmc.2022.89236
Sponholz M. (2021). Physical therapy and rehabilitation for Alzheimer’s and dementia. https://www.agingcare.com
Stanford Medicine. (2024a). Alzheimer’s disease. https://stanfordhealthcare.org
Stanford Medicine. (2024b). Laboratory tests for dementia. https://stanfordhealthcare.org
Staples W. (2021). Physical therapy guide to Alzheimer’s disease. https://www.choosept.com
Statista. (2024a). Distribution of Alzheimer’s disease among adults in the United States as of 2024, by age. https://www.statista.com
Statista. (2024b). Annual median cost of long-term health care services in the United States as of 2023, by type. https://www.statista.com
Statistics Solutions. (2024). Functional assessment questionnaire. https://www.statisticssolutions.com
Stromsdorfer S. (2022). OT interventions for dementia. https://www.myotspot.com
Taffet G. (2023). Normal aging. UpToDate. https://www.uptodate.com
Teepasnow.com. (2024). Radically transforming the experience of dementia. https://teepasnow.com
Thomas R & Reeves M. (2023). Mandatory reporting laws. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Treem J. (2023). Medical aid in dying: Ethical and practical issues. J Adv Pract Oncol, 14(3), 207–11. https://doi.org/10.6004/jadpro.2023.14.3.5
University of California, San Francisco (UCSF). (2024a). Memory. https://memory.ucsf.edu
University of California, San Francisco (UCSF). (2024b). Executive functions. https://memory.ucsf.edu
University of California, San Francisco (UCSF). (2024c). Behavior. https://memory.ucsf.edu
University of California, San Francisco (UCSF). (2024d). Behavior and personality changes. https://memory.ucsf.edu
University of California, San Francisco (UCSF). (2024e). Amyloid PET scan for Alzheimer’s disease assessment. https://radiology.ucsf.edu
University of Illinois. (2024). Modified interest checklist. https://moho-irm.uic.edu
University of Pittsburgh. (2024). Skills2Care: A personalized education experience for occupational therapy students to help older adults age in place. https://www.studentsuccess.pitt.edu
Van Dyke D & Dono L. (2023). 50 tips to help keep dementia and Alzheimer's patients safe in your home. https://www.aarp.org
Victoria Department of Health. (2022). Designing for people with dementia. https://www.health.vic.gov
Voss R & Das J. (2024). Mental status examination. StatPearls Publishing. https://www.ncbi.nlm.nih.gov
Wallensten J, Ljunggren G, Nager A, et al. (2023). Stress, depression, and risk of dementia – A cohort study in the total population between 18 and 65 years old in Region Stockholm. Alz Res Therapy, 15, 161. https://doi.org/10.1186/s13195-023-01308-4
Wiley R. (2023). Dementia and occupational therapy guide. https://otpotential.com
Wolk D & Dickerson B. (2024). Clinical features and diagnosis of Alzheimer disease. UpToDate. https://www.uptodate.com
World Health Organization (WHO). (2024). Dementia. https://www.who.int/health-topics/dementia#tab=tab_1
Zheng C, Zeng R, et al. (2024). Beyond vision: A view from eye to Alzheimer’s disease and dementia. J Prev Alzheimers Dis, 11, 469–83. https://doi.org/10.14283/jpad.2023.118






Customer Rating
4.9 / 499 ratings